Due to the extensive application and high efficiency feasibility of propylene in organic synthesis over the past few decades, the method of building compounds using propylene blocks has become increasingly popular.1 Its versatility is mainly manifested in its ability to participate in nucleophilic and electrophilic addition reactions, cycloaddition reactions, cyclization reactions, and various palladium- and gold- catalyzed organic reactions. In addition, the allene also exists in many biologically active natural compounds and pharmaceutical reagents.
Bolte and Gagosz completed the hydroalkylation of propylene under the catalytic conditions of gold (I) phosphite complex or Brønsted acid to produce various fused and spiro tetrahydrofurans and tetrahydropyrans (Scheme 1). The reaction catalyzed by Brønsted acid is carried out in a stereoselective manner under mild conditions, forming two adjacent asymmetric centers. Then, when using the gold (I) catalyst, the selectivity of the product is completely reversed, and the main product is fused. 2

Scheme 1.Hydroalkylation of allenyl-containing ethers
Poonoth and Krause have recently reported that under room temperature conditions, the cycloisomerization reaction of allenic hydroxyl ketones can occur in NaOH aqueous solution to form 3(2H)-furanone (Scheme 2).3 The reaction can proceed smoothly without heating or cooling, and no metal catalyst is required.

Scheme 2. Cycloisomerization of allenes to form 3(2H)-furanones
The Krische Research Group at the University of Texas at Austin reported on the asymmetric hydrohydroxyalkylation of 1,1-disubstituted allenes under ruthenium catalysis under various conditions, successfully achieving the construction of a quaternary stereocenter with anti-diastereoselectivity (Scheme 3).4 By analogy, a method for enantioselective synthesis of acrylates from propylene via catalytic [3+2] cycloaddition of allenes reaction was also designed to form a cyclic scaffold containing a quaternary stereocenter(Scheme 4).5 In addition, it was found that the O-TBDPS-D-Thr-L-tert-Leu derivative had better reaction effects than other variants through the cyclization of propylene catalyzed by various amino phosphines.
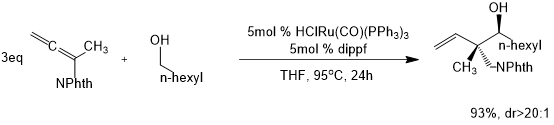
Scheme 3.Hydrohydroxyalkylation of 1,1-disubstituted allenes

Scheme 4. Enantioselective [3+2] cycloaddition of allenes to acrylates
Ryu and colleagues reported that in the presence of AIBN as a free radical initiator, the regioselective free radical bromoallylation of allenes can be effectively carried out, and 2-bromo-1,5-dienes can be obtained in excellent yields. The addition of bromine radicals occurs in a regioselective manner on the central carbon of allenes, producing stable allyl radicals, which underwent addition/β-fragmentation reactions with allylbromides.(Scheme 5). 6

Scheme 5. Generation of 2-bromo-1,5-dienes via radical bromoallylation
Under similar conditions, Kwon and his colleagues have also used triphenylphosphine catalyzed nucleophilic reagents to activate α-disubstituted allenes realizes β’- Umpolung addition to produce functionalized olefins with high stereoselectivity can provide many potentially useful synthesis intermediates (Scheme 6).7

Scheme 6. β’-Umpolung addition of nucleophiles to activated allenes
Fujii, Ohno, and colleagues recently disclosed the enantioselective total synthesis of (+)-lysergic acid and related indole alkaloids. The key feature of the synthesis is the Pd (0) catalyzed cyclization of dominoes containing amino and bromo indole partial allenes (Scheme 7).8 This cyclization can directly construct the C-ring and D-ring systems of the alkaloid skeleton, and the reaction proceeds with good diastereoselectivity (dr=92:8).

Scheme 7 .Pd(0)-catalyzed domino cyclization of allene in the synthesis of (+)-lysergic acid
References:
1.Selected recent reviews: (a) Ma, S. Aldrichimica Acta 2007, 40, 91. (b) Brummond, K. M.; Chen, H. In Modern Allene Chemistry; Krause, N., Hashmi, A. S. K., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Vol. 2, pp 1041-1089. (c) Aubert, C. et al. Chem. Rev. 2011, 111, 1954. (d) Zimmer, R. et al. Chem. Rev. 2000, 100, 3067. (e) Sydnes, L. Chem. Rev. 2003, 103, 1133. (f) Ma, S. Chem. Rev. 2005, 105, 2829. (g) Alcaide, B. et al. Chem. Soc. Rev. 2010, 39, 783. (h) Krause, N.; Winter, C. Chem. Rev. 2011, 111, 1994..
2.Bolte B, Gagosz F. 2011. Gold and Brønsted Acid Catalyzed Hydride Shift onto Allenes: Divergence in Product Selectivity. J. Am. Chem. Soc.. 133(20):7696-7699. https://doi.org/10.1021/ja202336p
3.Poonoth M, Krause N. 2011. Cycloisomerization of Bifunctionalized Allenes: Synthesis of 3(2H)-Furanones in Water. J. Org. Chem.. 76(6):1934-1936. https://doi.org/10.1021/jo102416e
4.Zbieg JR, McInturff EL, Leung JC, Krische MJ. 2011. Amplification of Anti-Diastereoselectivity via Curtin?Hammett Effects in Ruthenium-Catalyzed Hydrohydroxyalkylation of 1,1-Disubstituted Allenes: Diastereoselective Formation of All-Carbon Quaternary Centers. J. Am. Chem. Soc.. 133(4):1141-1144. https://doi.org/10.1021/ja1104156
5.Han X, Wang Y, Zhong F, Lu Y. 2011. Enantioselective [3 + 2] Cycloaddition of Allenes to Acrylates Catalyzed by Dipeptide-Derived Phosphines: Facile Creation of Functionalized Cyclopentenes Containing Quaternary Stereogenic Centers. J. Am. Chem. Soc.. 133(6):1726-1729. https://doi.org/10.1021/ja1106282
6.Kippo T, Fukuyama T, Ryu I. 2011. Regioselective Radical Bromoallylation of Allenes Leading to 2-Bromo-Substituted 1,5-Dienes. Org. Lett.. 13(15):3864-3867. https://doi.org/10.1021/ol201395p
7.Martin TJ, Vakhshori VG, Tran YS, Kwon O. 2011. Phosphine-Catalyzed β′-Umpolung Addition of Nucleophiles to Activated α-Alkyl Allenes. Org. Lett.. 13(10):2586-2589. https://doi.org/10.1021/ol200697m
8.Inuki S, Iwata A, Oishi S, Fujii N, Ohno H. 2011. Enantioselective Total Synthesis of (+)-Lysergic Acid, (+)-Lysergol, and (+)-Isolysergol by Palladium-Catalyzed Domino Cyclization of Allenes Bearing Amino and Bromoindolyl Groups. J. Org. Chem.. 76(7):2072-2083. https://doi.org/10.1021/jo102388e







 浙公网安备 33010802013016号
浙公网安备 33010802013016号