组分信息
| CAS号 | 名称 | 标准值 | 单位 |
|---|---|---|---|
| 74011-58-8 | 依诺沙星 | 100 | μg/mL |
| 79660-72-3 | 氟罗沙星 | 100 | μg/mL |
| 82419-36-1 | 氧氟沙星 | 100 | μg/mL |
| 70458-96-7 | 诺氟沙星 | 100 | μg/mL |
| 70458-92-3 | 培氟沙星 | 100 | μg/mL |
| 85721-33-1 | 环丙沙星 | 100 | μg/mL |
| 93106-60-6 | 恩诺沙星 | 100 | μg/mL |
| 91296-87-6 | 盐酸沙拉沙星 | 100 | μg/mL |
| 91296-86-5 | 盐酸二氟沙星 | 100 | μg/mL |
| 186826-86-8 | 盐酸莫西沙星 | 100 | μg/mL |
详细介绍
10种喹诺酮类药物混标/化妆品安全技术规范2015版 第四章 2.3/保质期9个月
This certificate is designed in accordance with ISO 17034 and ISO Guide 31. This reference material (RM) was designed,produced and verified in accordance with ISO/IEC 17025, ISO 17034 and a registered quality management system ISO 9001.
冷藏(2~8)℃,置于阴凉处
| CERTIFIED | |||||
|
Component No. (组分数) |
Concentration (μg/mL) (浓度) |
Relative Expanded Uncertainty(%) (k=2) (相对扩展不确定度) |
|||
| 1 | 1 | Enoxacin (依诺沙星) | 74011-58-8 | 100 | 3 |
| 2 | 2 | Fleroxacin (氟罗沙星) | 79660-72-3 | 100 | 3 |
| 3 | 3 | Ofloxacin (氧氟沙星) | 82419-36-1 | 100 | 3 |
| 4 | 4 | Norfloxacin (诺氟沙星) | 70458-96-7 | 100 | 3 |
| 5 | 5 | Pefloxacin (培氟沙星) | 70458-92-3 | 100 | 3 |
| 6 | 6 | Ciprofloxacin (环丙沙星) | 85721-33-1 | 100 | 3 |
| 7 | 7 | Enrofloxacin (恩诺沙星) | 93106-60-6 | 100 | 3 |
| 8 | 8 | Sarafloxacin Hydrochloride1 (盐酸沙拉沙星) | 91296-87-6 | 100(free base) | 3 |
| 9 | 9 | Difloxacin Hydrochloride1 (盐酸二氟沙星) | 91296-86-5 | 100(free base) | 3 |
| 10 | 10 | Moxifloxacin Hydrochloride1 (盐酸莫西沙星) | 186826-86-8 | 100(free base) | 3 |
This RM is intended for use in a laboratory as a calibration and quality control standard or in method development for analytical techniques.
The certified value(s) and uncertainty(ies) are determined in accordance with ISO 17034 with an 95% confidence level (k=2). Uncertainty is based on the Total Combined Uncertainty, including uncertainties of preparation, purity of neat materials, homogeneity, stability testing.
The balances used for gravimetric measurements are calibrated with weights traceable to the national standards. The calibration of the balances is verified annually by an external accredited calibration service. This analysis method has been verified using an approach consistent with ISO 17034:2016 & ISO 17025:2017.
Random replicate samples of the final packaged RM have been analysed to prove homogeneity consistent with ISO 17034.
The RM should be stored in the original sealed bottle at the indicated temperature.
| CERTIFICATE ON | QC SIGNATURE | |
| 2022-Dec-29 |

|
RM Release |
相关文档
化学品安全说明书(MSDS)
下载MSDS质检证书(COA)
相关产品
-
CAS号:14644-61-2
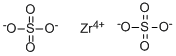
硫酸锆
¥164.00
-
CAS号:23007-85-4
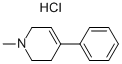
1-甲基-4-苯基-1,2,3...
¥0.00
-
CAS号:11013-97-1
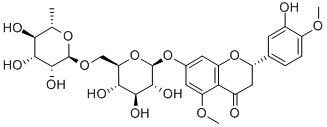
甲基橙皮苷
¥139.00
-
CAS号:24292-52-2
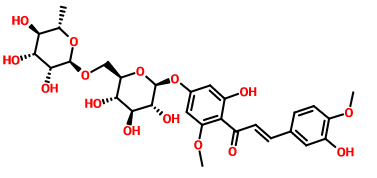
橙皮苷甲基查尔酮
¥70.00
-
CAS号:204259-66-5
1-溴十二烷-d25
¥500.00
-
CAS号:362472-81-9
新莪术二酮
¥2,500.00
-
CAS号:31410-07-8
1-甲基-3-烯丙基咪唑溴盐
¥77.00
-
CAS号:870-63-3
1-溴-3-甲基-2-丁烯
¥590.00
-
CAS号:54-71-7
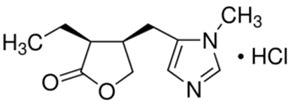
盐酸匹罗卡品
¥530.00
-
CAS号:141-52-6
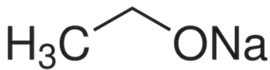
乙醇钠
¥480.00
-
CAS号:506-87-6
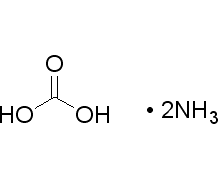
碳酸铵
¥120.00
-
CAS号:768-95-6
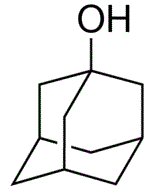
1-金刚烷醇
¥320.00






 浙公网安备 33010802013016号
浙公网安备 33010802013016号