| 产品介绍 |
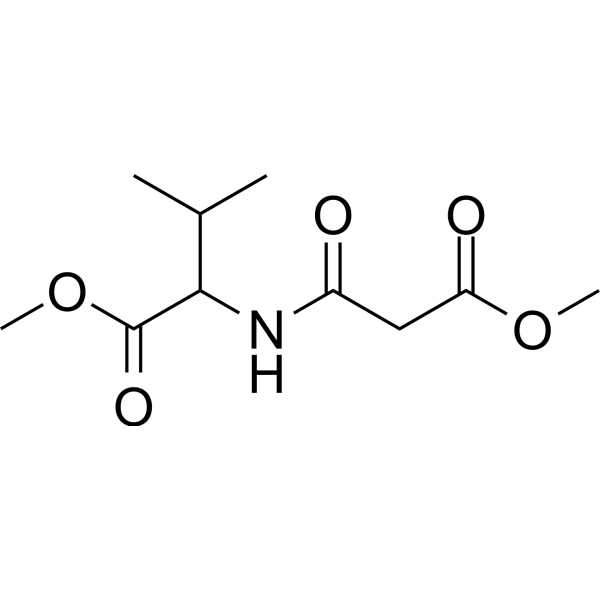
(Rac)-ZLc-002 是 nNOS 与一氧化氮合酶 1 衔接蛋白 (NOS1AP) 相互作用的抑制剂,抑制炎症性痛觉和化疗诱导的神经性疼痛,并与 Paclitaxel (HY-B0015) 协同降低肿瘤细胞活力。
|
|---|
| 生物活性 |
(Rac)-ZLc-002, an inhibitor of nNOS interaction with nitric oxide synthase 1 adaptor protein (NOS1AP), suppresses inflammatory nociception and chemotherapy-induced neuropathic pain and synergizes with Paclitaxel (HY-B0015) to reduce tumor cell viability.
|
|---|
| 体外研究 |
(Rac)-ZLc-002 (10 μM) 可降低原代培养皮层神经元 NOS1AP 与 nNOS 免疫共沉淀。(Rac)-ZLc-002 在 AlphaScreen 体外结合实验中未能破坏 nNOS-NOS1AP 蛋白-蛋白相互作用。在无细胞检测中无活性。
(Rac)-ZLc-002 (10 μM) 可降低共表达全长 nNOS 的 HEK293T 细胞中全长 NOS1AP 的共免疫沉淀,但不能降低 PSD95-PDZ2 的共免疫沉淀。
(Rac)-ZLc-002 (0-50 μM; 72 h) 与 Paclitaxel (HY-B0015) 协同降低肿瘤细胞活力。
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
Cell Viability Assay
| Cell Line: |
4T1 cells |
| Concentration: |
0-50 µM |
| Incubation Time: |
72 h |
| Result: |
Treatment alone had no effect on the viability. Showed synergistic effect with Paclitaxel (HY-B0015). |
|
|---|
体内研究
(In Vivo) |
(Rac)-ZLc-002 (10 mg/kg; i.p.; once or daily for 8 days) 减轻 Paclitaxel (HY-B0015) 引起的机械性和冷性异位痛,抑制 Paclitaxel 诱导的小鼠模型神经性疼痛。
(Rac)-ZLc-002 (4 and 10 mg/kg; i.p.; once) 降低大鼠脊髓背角福尔马林诱发的痛觉行为和福尔马林样免疫反应性。
(Rac)-ZLc-002 (40 mg/kg/day; i.v.; 7 days) 减弱 ICR 小鼠慢性轻度应激 (CMS) 诱导的焦虑行为。
(Rac)-ZLc-002 (10 μM; 1 μl; 30 min after Corticosterone, HY-B1618) 海马体注射 7 天逆转糖皮质激素对小鼠的行为影响。
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
Adult C57BL/6J male mice, paclitaxel model of neuropathic pain |
| Dosage: |
10 mg/kg |
| Administration: |
IP, once or daily for 8 days |
| Result: |
Increased postinjection mechanical paw withdrawal thresholds, mechanical paw withdrawal thresholds differed across postinjection times, and the interaction between drug treatment and injection time was significant. Elevated mechanical paw withdrawal thresholds relative to vehicle treatment from 30 min. Decreased postinjection cold responsiveness, cold responsiveness did not differ reliably across postinjection times, and the interaction between drug treatment and injection time was not significant. Once daily dosing increased mechanical paw withdrawal thresholds relative to the vehicle-treated group across the observation interval.
|
|
| 体内研究 |
(Rac)-ZLc-002 (10 mg/kg; i.p.; once or daily for 8 days) 减轻 Paclitaxel (HY-B0015) 引起的机械性和冷性异位痛,抑制 Paclitaxel 诱导的小鼠模型神经性疼痛。
(Rac)-ZLc-002 (4 and 10 mg/kg; i.p.; once) 降低大鼠脊髓背角福尔马林诱发的痛觉行为和福尔马林样免疫反应性。
(Rac)-ZLc-002 (40 mg/kg/day; i.v.; 7 days) 减弱 ICR 小鼠慢性轻度应激 (CMS) 诱导的焦虑行为。
(Rac)-ZLc-002 (10 μM; 1 μl; 30 min after Corticosterone, HY-B1618) 海马体注射 7 天逆转糖皮质激素对小鼠的行为影响。
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
Adult C57BL/6J male mice, paclitaxel model of neuropathic pain |
| Dosage: |
10 mg/kg |
| Administration: |
IP, once or daily for 8 days |
| Result: |
Increased postinjection mechanical paw withdrawal thresholds, mechanical paw withdrawal thresholds differed across postinjection times, and the interaction between drug treatment and injection time was significant. Elevated mechanical paw withdrawal thresholds relative to vehicle treatment from 30 min. Decreased postinjection cold responsiveness, cold responsiveness did not differ reliably across postinjection times, and the interaction between drug treatment and injection time was not significant. Once daily dosing increased mechanical paw withdrawal thresholds relative to the vehicle-treated group across the observation interval.
|
|
|---|
| 体内研究 |
(Rac)-ZLc-002 (10 mg/kg; i.p.; once or daily for 8 days) 减轻 Paclitaxel (HY-B0015) 引起的机械性和冷性异位痛,抑制 Paclitaxel 诱导的小鼠模型神经性疼痛。
(Rac)-ZLc-002 (4 and 10 mg/kg; i.p.; once) 降低大鼠脊髓背角福尔马林诱发的痛觉行为和福尔马林样免疫反应性。
(Rac)-ZLc-002 (40 mg/kg/day; i.v.; 7 days) 减弱 ICR 小鼠慢性轻度应激 (CMS) 诱导的焦虑行为。
(Rac)-ZLc-002 (10 μM; 1 μl; 30 min after Corticosterone, HY-B1618) 海马体注射 7 天逆转糖皮质激素对小鼠的行为影响。
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
Adult C57BL/6J male mice, paclitaxel model of neuropathic pain |
| Dosage: |
10 mg/kg |
| Administration: |
IP, once or daily for 8 days |
| Result: |
Increased postinjection mechanical paw withdrawal thresholds, mechanical paw withdrawal thresholds differed across postinjection times, and the interaction between drug treatment and injection time was significant. Elevated mechanical paw withdrawal thresholds relative to vehicle treatment from 30 min. Decreased postinjection cold responsiveness, cold responsiveness did not differ reliably across postinjection times, and the interaction between drug treatment and injection time was not significant. Once daily dosing increased mechanical paw withdrawal thresholds relative to the vehicle-treated group across the observation interval.
|
|
|---|
| 性状 | Solid |
|---|
| 溶解性数据 | |
|---|
| 运输条件 |
Room temperature in continental US; may vary elsewhere.
|
|---|
| 储存方式 |
| Powder |
-20°C |
3 years |
|
4°C |
2 years |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
|
|---|
| 参考文献 | |
|---|


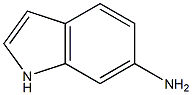
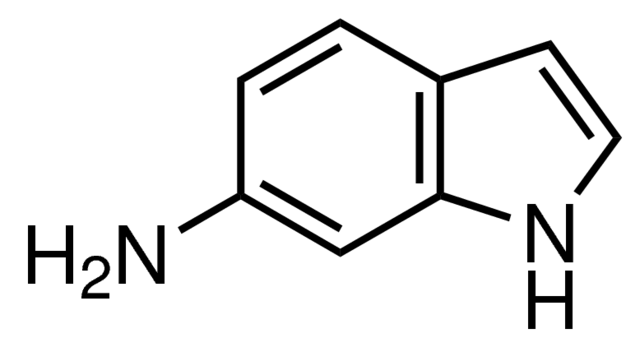
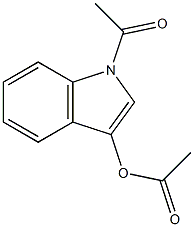
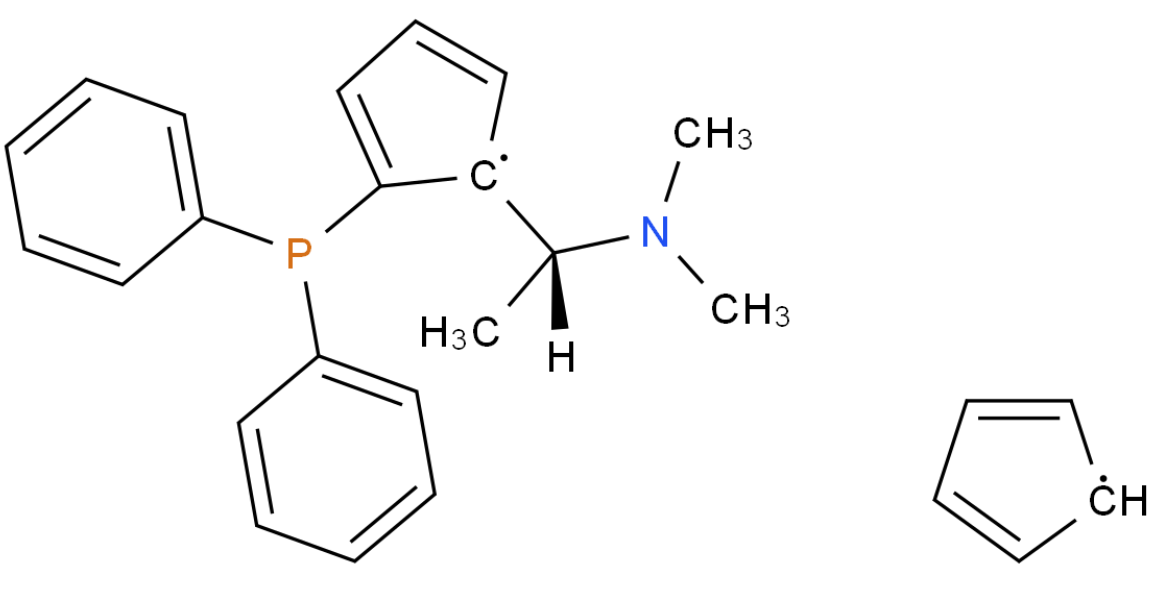





 浙公网安备 33010802013016号
浙公网安备 33010802013016号