Risedronic acid 利塞膦酸; Risedronate,98.0%
产品编号:Bellancom-B0148| CAS NO:105462-24-6| 分子式:C7H11NO7P2| 分子量:283.11
Risedronic acid(Risedronate)能抑制破骨细胞介导的骨重吸收。
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Risedronic acid 利塞膦酸; Risedronate
| 产品介绍 | Risedronic acid(Risedronate)能抑制破骨细胞介导的骨重吸收。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Risedronic acid (Risedronate ) is a pyridinyl biphosphonate which inhibits osteoclast-mediated bone resorption. Target: Others Risedronate, which was promoted in Croatia a few months ago, is the latest (III) generation of bisphosphonates, the most efficient anti-resorption drugs that inhibit osteoclast-mediated bone resorption and change the bone metabolism. Risedronate is hence the first line of bisphosphonates for the reduction of vertebral and non-vertebral fracture risks in postmenopausal women with osteoporosis or those with a high risk of osteoporosis. It also efficiently prevents bone loss or improves bone density in men and women on a long-term corticosteroid therapy . The administration of 20 and 25 mg/kg risedronate for 4 days led to decreases of parasitemia of 68.9% and 83.6%, respectively. On the seventh day of treatment the inhibitions were 63% and 88.9% with 20 and 25 mg/kg, respectively. After recovering the parasitemia, a dose-response curve was obtained for estimating the ID50 (dose causing 50% inhibition), equivalent to 17 ± 1.8 mg/kg after 7 days of treatment. Four days after the interruption of treatment (11 days postinfection), the parasitemias of the groups treated with 10, 15, 20, and 25 mg/kg/day were 15.3%, 15.9%, 15.2%, and 5.7%, respectively. Conversely, the group that received PBS presented parasitemia of 25.6%. Among the groups treated with risedronate, only the animals that received 25 mg/kg had a significant inhibition of 77.8% (see Table S1 in the supplemental material), demonstrating that even after treatment discontinuation, the parasitemia of the animals remained low in relation to that of the controls . | ||||||||||||||||
| 体外研究 | |||||||||||||||||
| 体内研究 | |||||||||||||||||
| 体内研究 | |||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
0.1 M NaOH : 11 mg/mL (38.85 mM; ultrasonic and adjust pH to 7 with NaOH) H2O : 0.67 mg/mL (2.37 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 | ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |
| ||||||||||||||||
| 海关编码 | 2933399090 |
|---|
|
~98% 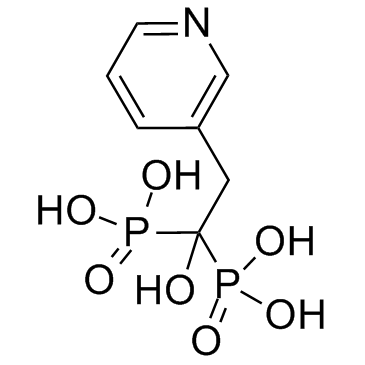
105462-24-6 |
| 文献:JUBILANT ORGANOSYS LIMITED Patent: WO2006/51553 A1, 2006 ; Location in patent: Page/Page column 8 ; |
|
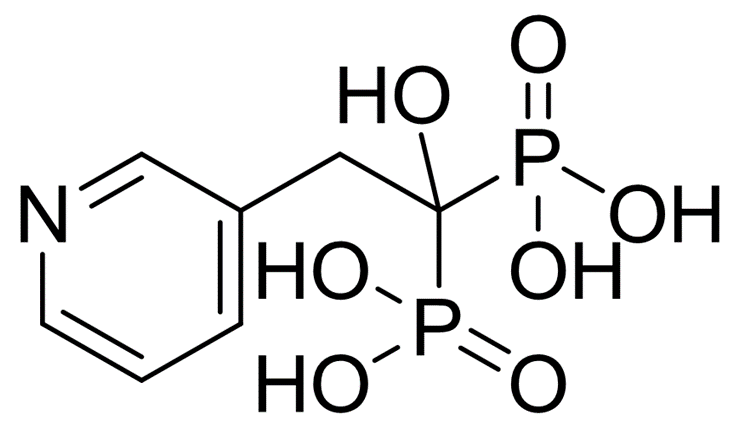
~81% 
105462-24-6 |
| 文献:FLEMING LABORATORIES LIMITED Patent: WO2009/34580 A1, 2009 ; Location in patent: Page/Page column 7 ; |
|
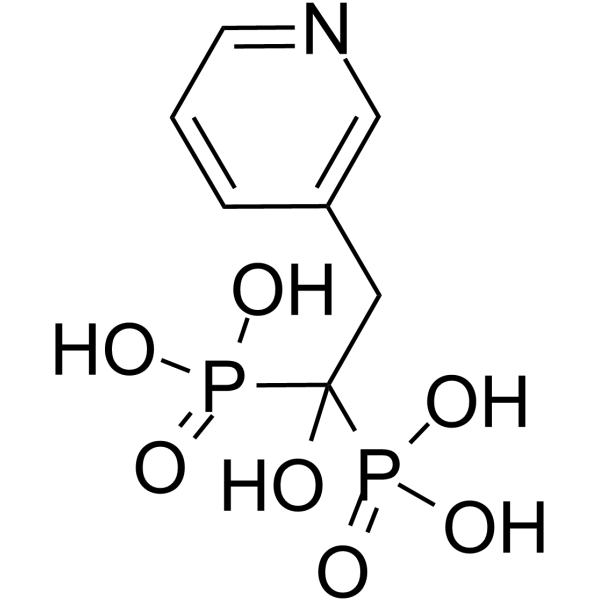
~82% 
105462-24-6 |
| 文献:ZAKLADY FARMACEUTYCZNE S.A.; POLITECHNIKA GDANSKA Patent: WO2006/71128 A1, 2006 ; Location in patent: Page/Page column 4; 5 ; |
|
~% 
105462-24-6 |
| 文献:WO2008/58722 A1, ; Page/Page column 11-12 ; |
|
~% 
105462-24-6 |
| 文献:WO2008/44245 A2, ; Page/Page column 5 ; |
|
~% 
105462-24-6 |
| 文献:US5391743 A1, ; |
|
~% 
105462-24-6 |
| 文献:Phosphorus, Sulfur and Silicon and the Related Elements, , vol. 188, # 1-3 p. 39 - 41 |
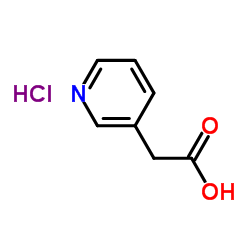
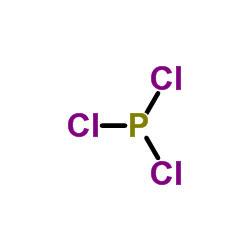
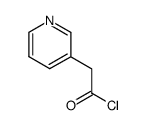
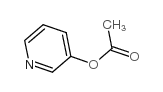
| 上游产品 6 | |
|---|---|
| 下游产品 0 | |
 有竞争力的价格
有竞争力的价格匹配竞争对手的价格
 极速物流
极速物流效率为先
 技术支持
技术支持专业经验 贴心服务
 现货库存
现货库存50000+库存


 浙公网安备 33010802013016号
浙公网安备 33010802013016号