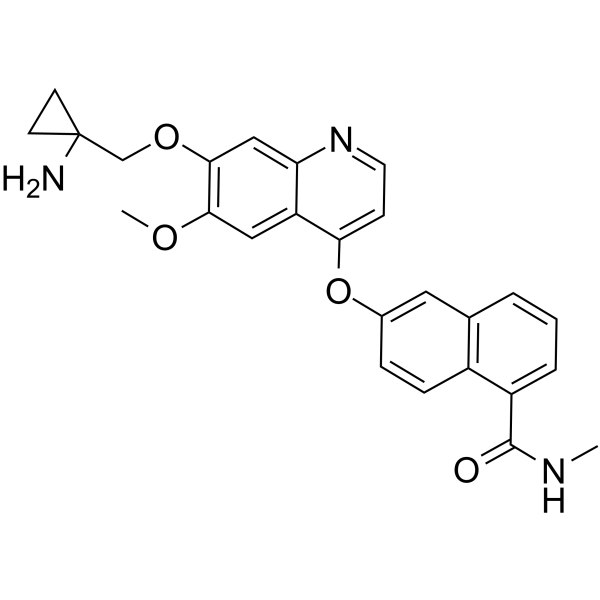
Lucitanib 德立替尼; E-3810,98.94%
产品编号:Bellancom-15391| CAS NO:1058137-23-7| 分子式:C26H25N3O4| 分子量:443.49
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Lucitanib 德立替尼; E-3810
| 产品介绍 | Lucitanib (E-3810) 是 VEGFR 和 FGFR 的双重抑制剂,有效和选择性地抑制VEGFR1,VEGFR2,VEGFR3,FGFR1,FGFR2,IC50分别为7 nM,25 nM,10 nM,17.5 nM,82.5 nM。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Lucitanib (E-3810) is a novel dual inhibitor of VEGFR and FGFR, potently and selectively inhibits VEGFR1, VEGFR2, VEGFR3, FGFR1 and FGFR2 with IC50s of 7 nM, 25 nM, 10 nM, 17.5 nM, and 82.5 nM, respectively. | ||||||||||||||||
| 体外研究 |
Consistent with the inhibitory activity of VEGFR and FGFR auto-phosphorylation, Lucitanib potently inhibits VEGF and bFGF-stimulated HUVEC proliferation with IC50 of 40 and 50 nM, respectively. Besides, Lucitanib (E-3810) also inhibits CSF-1R with IC50 of 5 nM. Lucitanib potently inhibits FGFR2 activity (Ki<0.05 μM), follows by PDGFRα activity (Ki=0.11 μM). The Ki values obtained for DDR2, LYN, CARDIAK, CSBP (2), EPHA2, and YES range between 0.26 and 8 μM. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Lucitanib (E-3810), at oral dosing of 20 mg/kg for 7 consecutive days, completely inhibits (P<0.01) the bFGF induced angiogenic response compare with the response in vehicle-treated mice. Lucitanib (E-3810) shows a broad spectrum of activity, being active in all the xenografts tested (HT29 colon carcinoma, A2780 ovarian carcinoma, A498, SN12K1, and RXF393 renal carcinomas) with dose-dependent inhibition of tumor growth. E-3810 significantly delays growth during treatment, but tumors resume their growth when treatment is suspended; in a few cases, tumor regression is observed. The activity of Lucitanib (E-3810) given at the doses of 15 mg/kg is tested on MDA-MB-231 breast cancer transplanted subcutaneously, at a late stage, when tumor masses reach 350 to 400 mg. This tumor xenograft is very sensitive to Lucitanib (E-3810), with complete tumor stabilization lasting throughout the 30-day treatment. As in other tumor models, tumors re-grow after withdrawal of Lucitanib (E-3810) at a rate similar to control tumors. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Lucitanib (E-3810), at oral dosing of 20 mg/kg for 7 consecutive days, completely inhibits (P<0.01) the bFGF induced angiogenic response compare with the response in vehicle-treated mice. Lucitanib (E-3810) shows a broad spectrum of activity, being active in all the xenografts tested (HT29 colon carcinoma, A2780 ovarian carcinoma, A498, SN12K1, and RXF393 renal carcinomas) with dose-dependent inhibition of tumor growth. E-3810 significantly delays growth during treatment, but tumors resume their growth when treatment is suspended; in a few cases, tumor regression is observed. The activity of Lucitanib (E-3810) given at the doses of 15 mg/kg is tested on MDA-MB-231 breast cancer transplanted subcutaneously, at a late stage, when tumor masses reach 350 to 400 mg. This tumor xenograft is very sensitive to Lucitanib (E-3810), with complete tumor stabilization lasting throughout the 30-day treatment. As in other tumor models, tumors re-grow after withdrawal of Lucitanib (E-3810) at a rate similar to control tumors. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 20.83 mg/mL (46.97 mM) * "≥" means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |
|






 浙公网安备 33010802013016号
浙公网安备 33010802013016号