| 产品介绍 |
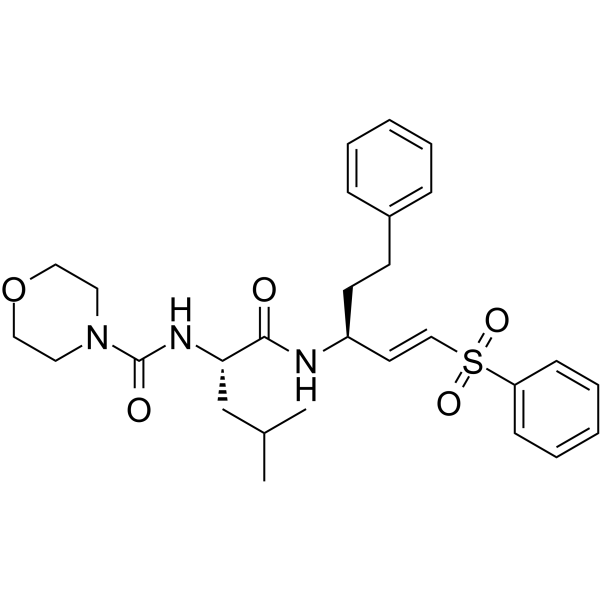
LHVS 是一种有效的,非选择性的,不可逆的,可透过细胞的半胱氨酸蛋白酶 (cysteine protease) 和组织蛋白酶 (cathepsin) 抑制剂。LHVS 可减少肌动蛋白环的形成。LHVS 抑制 T. gondii 侵袭,其 IC50 为 10 μM。
|
|---|
| 生物活性 |
LHVS is a potent, non-selective, irreversible, cell-permeable cysteine protease and cathepsin inhibitor. LHVS decreases actin ring formation. LHVS inhibits T. gondii invasion with an IC50 of 10 μM.
|
|---|
| 体外研究 |
LHVS (5 μM, 2 h) results in a 50% reduction of actin ring formation in wild-type osteoclasts when compared with untreated osteoclasts.
LHVS acts in a dose-dependent manner on osteoclasts and at 5 μM, LHVS inhibits cathepsins K, L, S, and B.
LHVS (1-5 nM) can inhibit specifically cathepsin S in HOM2 cells, leaving other cysteine proteases functionally active.
LHVS impairs tachyzoite attachment by blocking the release of at least two key invasion proteins, MIC2 and M2AP, from the micronemes.
LHVS (50 μM) selectively impairs microneme protein secretion.
西域 has not independently confirmed the accuracy of these methods. They are for reference only. |
|---|
| 体内研究 |
LHVS (3-30 mg/kg, SC, once) shows anti-hyperalgesic effect in neuropathic rats[4].
LHVS (30 nmol per rat, spinal delivery, daily) is antinociceptive in neuropathic rats[5].
LHVS (1-50 nmol per rat, Intrathecal injection, daily) reverses established neuropathic mechanical hyperalgesia in 14-day neuropathic rats[5].
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
Male Wistar rats (180-220 g)[4] |
| Dosage: |
3-30 mg/kg |
| Administration: |
SC, once |
| Result: |
Produced a dose-dependent reversal of the mechanical hyperalgesia which lasted up to 3 h in neuropathic rats. In contrast, a single systemic administration of LHVS did not reverse mechanical allodynia in neuropathic rats. |
| Animal Model: |
Male Wistar rats received a partial ligation of the left sciatic nerve (PNL)[5] |
| Dosage: |
30 nmol per rat |
| Administration: |
Spinal delivery, Daily |
| Result: |
Failed to prevent the development of allodynia when continuous delivery from day 0 to day 7 post-PNL, but significantly reversed allodynia on day 7 post-PNL. In addition, the delivery of LHVS from day 7 to day 14 post-PNL significantly reversed established mechanical allodynia from day 8. |
| Animal Model: |
Male Wistar rats received a partial ligation of the left sciatic nerve (PNL)[5] |
| Dosage: |
1, 10 or 50 nmol per rat |
| Administration: |
Intrathecal injection, Daily |
| Result: |
Reduced established mechanical hyperalgesia. This effect was dose-dependent and remained significant until 3 h after administration of the highest dose. |
|
|---|
| 体内研究 |
LHVS (3-30 mg/kg, SC, once) shows anti-hyperalgesic effect in neuropathic rats[4].
LHVS (30 nmol per rat, spinal delivery, daily) is antinociceptive in neuropathic rats[5].
LHVS (1-50 nmol per rat, Intrathecal injection, daily) reverses established neuropathic mechanical hyperalgesia in 14-day neuropathic rats[5].
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
Male Wistar rats (180-220 g)[4] |
| Dosage: |
3-30 mg/kg |
| Administration: |
SC, once |
| Result: |
Produced a dose-dependent reversal of the mechanical hyperalgesia which lasted up to 3 h in neuropathic rats. In contrast, a single systemic administration of LHVS did not reverse mechanical allodynia in neuropathic rats. |
| Animal Model: |
Male Wistar rats received a partial ligation of the left sciatic nerve (PNL)[5] |
| Dosage: |
30 nmol per rat |
| Administration: |
Spinal delivery, Daily |
| Result: |
Failed to prevent the development of allodynia when continuous delivery from day 0 to day 7 post-PNL, but significantly reversed allodynia on day 7 post-PNL. In addition, the delivery of LHVS from day 7 to day 14 post-PNL significantly reversed established mechanical allodynia from day 8. |
| Animal Model: |
Male Wistar rats received a partial ligation of the left sciatic nerve (PNL)[5] |
| Dosage: |
1, 10 or 50 nmol per rat |
| Administration: |
Intrathecal injection, Daily |
| Result: |
Reduced established mechanical hyperalgesia. This effect was dose-dependent and remained significant until 3 h after administration of the highest dose. |
|
|---|
| 性状 | Solid |
|---|
| 溶解性数据 |
In Vitro:
DMSO : 100 mg/mL (189.51 mM; Need ultrasonic)
配制储备液
|
浓度
溶剂体积
质量
|
1 mg |
5 mg |
10 mg |
| 1 mM |
1.8951 mL |
9.4754 mL |
18.9509 mL |
| 5 mM |
0.3790 mL |
1.8951 mL |
3.7902 mL |
| 10 mM |
0.1895 mL |
0.9475 mL |
1.8951 mL |
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。
储备液的保存方式和期限:-80°C, 6 months; -20°C, 1 month。-80°C 储存时,请在 6 个月内使用,-20°C 储存时,请在 1 个月内使用。
In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
分比是指该溶剂在您配制终溶液中的体积占比;如在配制过程中出现沉淀、析出现象,可以通过加热和/或超声的方式助溶
-
1.
请依序添加每种溶剂: 10% DMSO 40% PEG300 5% Tween-80 45% saline Solubility: ≥ 2.5 mg/mL (4.74 mM); Clear solution
此方案可获得 ≥ 2.5 mg/mL (4.74 mM,饱和度未知) 的澄清溶液。 以 1 mL 工作液为例,取 100 μL 25.0 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀;向上述体系中加入50 μL Tween-80,混合均匀;然后继续加入 450 μL生理盐水定容至 1 mL。
-
2.
请依序添加每种溶剂: 10% DMSO 90% (20% SBE-β-CD in saline) Solubility: 2.5 mg/mL (4.74 mM); Suspended solution; Need ultrasonic
此方案可获得 2.5 mg/mL (4.74 mM) 的均匀悬浊液,悬浊液可用于口服和腹腔注射。 以 1 mL 工作液为例,取 100 μL 25.0 mg/mL 的澄清 DMSO 储备液加到 900 μL 20% 的 SBE-β-CD 生理盐水水溶液中,混合均匀。
-
3.
请依序添加每种溶剂: 10% DMSO 90% corn oil Solubility: ≥ 2.5 mg/mL (4.74 mM); Clear solution
此方案可获得 ≥ 2.5 mg/mL (4.74 mM,饱和度未知) 的澄清溶液,此方案不适用于实验周期在半个月以上的实验。 以 1 mL 工作液为例,取 100 μL 25.0 mg/mL 的澄清 DMSO 储备液加到 900 μL玉米油中,混合均匀。
*以上所有助溶剂都可在 西域 网站选购。
|
|---|
| 运输条件 |
Room temperature in continental US; may vary elsewhere.
|
|---|
| 储存方式 |
| Powder |
-20°C |
3 years |
|
4°C |
2 years |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
|
|---|
| 参考文献 | |
|---|






 浙公网安备 33010802013016号
浙公网安备 33010802013016号