Ranirestat AS-3201,98.32%
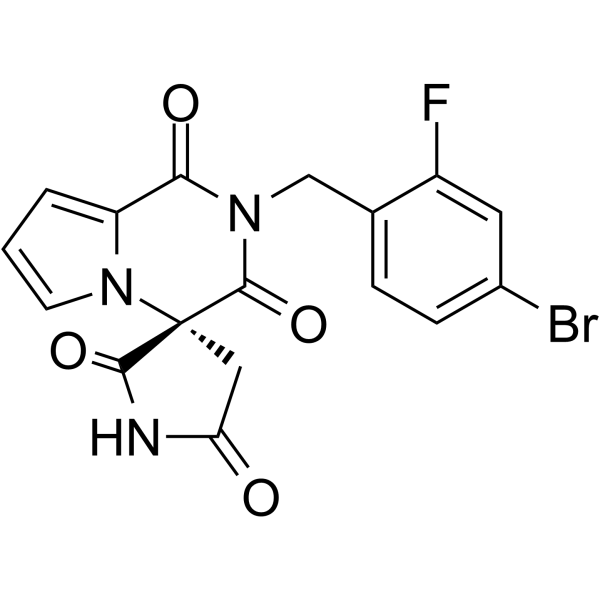
产品编号:Bellancom-15314| CAS NO:147254-64-6| 分子式:C17H11BrFN3O4| 分子量:420.19
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Ranirestat AS-3201
| 产品介绍 | Ranirestat (AS-3201) 是一种有效的,口服活性的醛糖还原酶 (AR) 抑制剂,对大鼠晶状体 AR 和重组人 AR 的 IC50 分别为 11 nM 和 15 nM,对于重组人 AR 的 Ki 为 0.38 nM。Ranirestat 可用于糖尿病感觉运动性多发性神经病的研究,并且对糖尿病视网膜具有神经保护作用。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Ranirestat (AS-3201) potent and orally active aldose reductase (AR) inhibitor with IC50s of 11 nM and 15 nM for rat lens AR and recombinant human AR, respectively, and a Ki of 0.38 nM for recombinant human AR. Ranirestat has the potential for diabetic sensorimotor polyneuropathy treatment. Ranirestat also has a neuroprotective effect on diabetic retinas. | ||||||||||||||||
| 体外研究 |
Ranirestat concentration-dependently inhibits sorbitol accumulation in rat erythrocytes and sciatic nerves incubated in the high concentration (500 mg/dl) of glucose. The potency of Ranirestat inhibition of sorbitol accumulation is similar between rat erythrocytes and sciatic nerves with IC50 values of 0.010 μM and 0.041 μM, respectively. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Ranirestat (0.03-1.0 mg/kg; oral administration; once daily; for 3 weeks; male STD-Wistar rats) treatment dose-dependently decreases the elevated sorbitol and fructose levels in the rat sciatic nerves without affecting blood glucose level. Ranirestat also improves the STZ-induced decrease in motor nerve conduction velocity (MNCV) in a dose-dependent manner. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 体内研究 |
Ranirestat (0.03-1.0 mg/kg; oral administration; once daily; for 3 weeks; male STD-Wistar rats) treatment dose-dependently decreases the elevated sorbitol and fructose levels in the rat sciatic nerves without affecting blood glucose level. Ranirestat also improves the STZ-induced decrease in motor nerve conduction velocity (MNCV) in a dose-dependent manner. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 50 mg/mL (118.99 mM) * "≥" means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |
|






 浙公网安备 33010802013016号
浙公网安备 33010802013016号