JNJ-18038683,99.76%
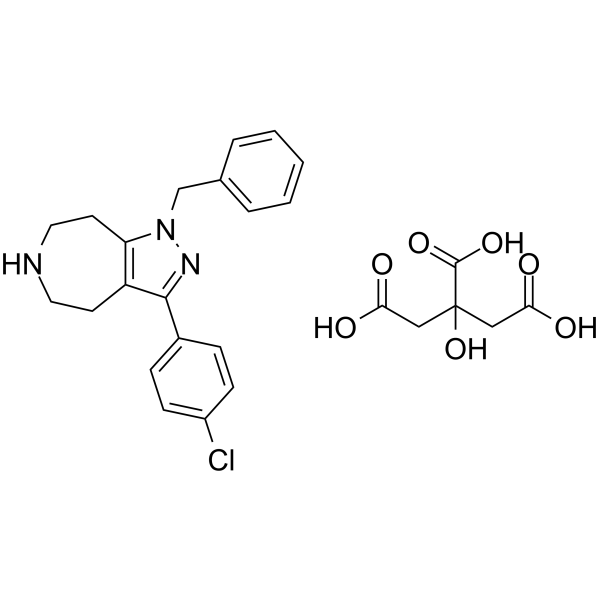
产品编号:Bellancom-19889| CAS NO:851376-05-1| 分子式:C26H28ClN3O7| 分子量:529.97
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
JNJ-18038683
| 产品介绍 | JNJ-18038683 是 5-羟色胺受体 7 (5-HT7) 的拮抗剂,HEK293 细胞中检测出其对大鼠和人类 5-羟色胺受体 7 的 pKi 值分别为 8.19,8.20。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | JNJ-18038683 is a 5-Hydroxytryptamine Type 7 (5-HT7) receptor antagonist, with pKis of 8.19, 8.20 for rat and human 5-HT7 in HEK293 cells, respectively. | ||||||||||||||||
| 体外研究 |
JNJ-18038683 displaced, with high affinity, specific [3H]5-CT binding sites from rat and human 5-HT7 receptor express in HEK293 cells (pKi=8.19±0.02 and 8.20±0.01, respectively). Similar values are obtained on the native 5-HT7 in membranes from rat thalamus (pKi=8.50±0.20). Hill slope values are close to unity, suggesting one-site competitive binding. Antagonist potency of JNJ-18038683 is determined by the measurement of adenylate cyclase activity in HEK293 cells expressing the human or rat 5-HT7 receptor. 5-HT stimulates adenylyl cyclase activity in rat and human 5-HT7/HEK293 cells with a pEC50 of 8.09 and 8.12, respectively. JNJ-18038683 produces a concentration-dependent decrease of 5-HT (100 nM)-stimulated adenylyl cyclase. The pKB values determined for JNJ-18038683 are in good agreement with the corresponding Ki values determined from [3H]5-CT binding studies. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
JNJ-18038683 dose-dependently suppresses REM sleep mainly during the first 4 h after the treatment. The duration of REM sleep is significantly decreased from the dose of 1 mg/kg onward (P<0.05) during the first 4 h after oral administration. Concomitantly, the REM sleep latency tends to be prolonged in a dose-related manner with a significant increase in REM latency occurring only at the highest dose tested (10 mg/kg; P<0.05). These alterations in REM sleep seem to be state-specific. A separate study is conducted to determine whether repeated administration of JNJ-18038683 for 7 days would result in an adaptation of the EEG sleep response in particular on REM sleep in rats during the course of the treatment and after its discontinuation. JNJ-18038683 is administered for 7 consecutive days (1 mg/kg s.c. per day) at 2 h into the light phase. On the first day of treatment, JNJ-18038683 produces a significant decrease in the time spent in REM sleep during the first 8 h after the injection and a prolongation of the REM sleep latency. The REM sleep latency is increased during the 7-day repeated treatment and is normalized on the first recovery day after cessation of treatment. The significant decrease in REM sleep time is maintained during the 7-day repeated treatment, with a rebound occurring on the first recovery day after treatment discontinuation. The NREM sleep latency and the total NREM sleep time are not affected during the entire treatment. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
JNJ-18038683 dose-dependently suppresses REM sleep mainly during the first 4 h after the treatment. The duration of REM sleep is significantly decreased from the dose of 1 mg/kg onward (P<0.05) during the first 4 h after oral administration. Concomitantly, the REM sleep latency tends to be prolonged in a dose-related manner with a significant increase in REM latency occurring only at the highest dose tested (10 mg/kg; P<0.05). These alterations in REM sleep seem to be state-specific. A separate study is conducted to determine whether repeated administration of JNJ-18038683 for 7 days would result in an adaptation of the EEG sleep response in particular on REM sleep in rats during the course of the treatment and after its discontinuation. JNJ-18038683 is administered for 7 consecutive days (1 mg/kg s.c. per day) at 2 h into the light phase. On the first day of treatment, JNJ-18038683 produces a significant decrease in the time spent in REM sleep during the first 8 h after the injection and a prolongation of the REM sleep latency. The REM sleep latency is increased during the 7-day repeated treatment and is normalized on the first recovery day after cessation of treatment. The significant decrease in REM sleep time is maintained during the 7-day repeated treatment, with a rebound occurring on the first recovery day after treatment discontinuation. The NREM sleep latency and the total NREM sleep time are not affected during the entire treatment. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : 200 mg/mL (377.38 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
4°C, sealed storage, away from moisture *In solvent : -80°C, 6 months; -20°C, 1 month (sealed storage, away from moisture) | ||||||||||||||||
| 参考文献 |






 浙公网安备 33010802013016号
浙公网安备 33010802013016号