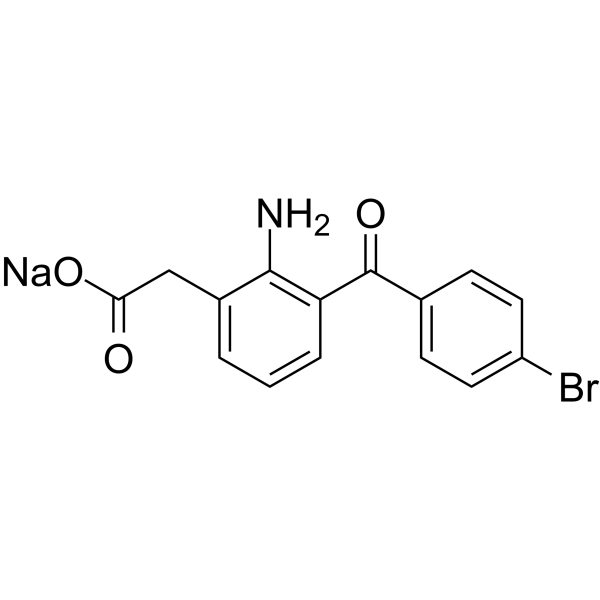
Bromfenac sodium 溴芬酸钠,98.52%
产品编号:Bellancom-B1888A| CAS NO:91714-93-1| 分子式:C15H11BrNNaO3| 分子量:356.15
Bromfenac sodium 是一种有效的和具有口服活性的 COX 抑制剂,抑制 COX-1 和 COX-2 的 IC50 值分别为 5.56 和 7.45 nM。Bromfenac sodium 是一种溴化非甾体类抗炎/镇痛药 (NSAID),通常用于白内障手术后的术后炎症和疼痛以及假晶状体囊状黄斑水肿 (CME) 的研究。
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Bromfenac sodium 溴芬酸钠
| 产品介绍 | Bromfenac sodium 是一种强效且具有口服活性的 COX 抑制剂,对 COX-1 和 COX-2 的 IC50 分别为 5.56 和 7.45 nM。Bromfenac sodium 可用于眼部炎症研究。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Bromfenac sodium is a potent and orally active inhibitor of COX, with IC50s of 5.56 and 7.45 nM for COX-1 and COX-2, respectively. Bromfenac sodium can be used in ocular inflammation research. | ||||||||||||||||
| 体外研究 |
Bromfenac (0-80 μg/mL; 24 h) can inhibit transforming growth factor-β2-induced epithelial-mesenchymal transition in HLEC-B3 in a concentration-dependent manner. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. Cell Viability Assay
Cell Migration Assay
|
||||||||||||||||
| 体内研究 (In Vivo) |
Bromfenac (0.0032-3.16%; 100 or 200 μL; rubbed onto the backs) produces significant anti-inflammatory activity at concentrations as low as 0.1% (4 h pretreatment time) or 0.32% (18 h pretreatment time) in rats. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 体内研究 |
Bromfenac (0.0032-3.16%; 100 or 200 μL; rubbed onto the backs) produces significant anti-inflammatory activity at concentrations as low as 0.1% (4 h pretreatment time) or 0.32% (18 h pretreatment time) in rats. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 体内研究 |
Bromfenac (0.0032-3.16%; 100 or 200 μL; rubbed onto the backs) produces significant anti-inflammatory activity at concentrations as low as 0.1% (4 h pretreatment time) or 0.32% (18 h pretreatment time) in rats. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : 11.36 mg/mL (31.90 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
4°C, sealed storage, away from moisture and light *In solvent : -80°C, 6 months; -20°C, 1 month (sealed storage, away from moisture and light) | ||||||||||||||||
| 参考文献 |
| ||||||||||||||||
| 符号 |
GHS09 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H400 |
| 警示性声明 | P273 |
| 危害码 (欧洲) | N |
| 风险声明 (欧洲) | 50 |
| 安全声明 (欧洲) | 61 |
| 危险品运输编码 | UN 3077 9 / PGIII |
| 产品名: | Bromfenac Sodium |
| CAS号: | 91714-93-1 |
| 制造商/供应商: | 西域试剂 网站:www.hzbp.cn 邮件:13911702513@139.com |
2. 合成/成分数据
| 产品名: | Bromfenac Sodium |
| 分子式: | C15H11BrNO3.Na |
| 分子量: | 356.15 |
3. 急救措施
| 吸入后: | 如果吸入,移至空气新鲜处,如果呼吸困难,给输氧,如呼吸停止,给予人工呼吸。 |
| 皮肤接触后: | 用大量的水冲洗,移除污染的衣服和鞋子。 |
| 眼睛接触后: | 检查并取下隐形眼镜,并用大量的水冲洗;呼叫医生。 |
| 吞食后: | 如果吞食,用大量纯净水漱口;呼叫医生。 |
4. 消防措施
| 适当的灭火剂: | 雾状水,二氧化碳,干粉或泡沫。 |
| 防护设备: | 穿戴自给式呼吸器和防护服,以防止与皮肤和眼睛接触。 |
5. 泄漏应急处理
| 安全防范措施: | 封锁泄漏区域;穿戴自给式呼吸器,防护服和厚橡胶手套。 |
| 清洁/收集措施: | 使用液体粘合原料(硅藻土,通用粘合剂)吸取精细粉末; 使用酒精擦洗表面和设备除去污渍; 根据第11条处理被污染的材料。 |
6. 处理和储存
| 安全处理说明: | 避免吸入和接触皮肤,眼睛及衣物;材料可能略微具有刺激性。 |
| 储存: |
粉末型式 -20°C 3年;4°C 2年 溶于溶剂 -80°C 6个月;-20°C 1个月 |
7. 接触控制和个人防护
| 呼吸设备: | NIOSH / MSHA认可的呼吸器。 |
| 双手保护: | 耐化学腐蚀的橡胶手套。 |
| 眼睛防护: | 化学安全护目镜。 |
8. 稳定性和反应活性
| 稳定性: | 按照说明存储是稳定的;避免强氧化剂。 |
| 热分解/其他要避免的情况: | 避免光和热。 |
9. 毒性资料
| 急性毒性: | 无可用资料。 |
| 主要刺激性影响: | 无可用资料。 |
| 在皮肤上: | 无可用资料。 |
| 对眼睛: | 无可用资料;可能具有刺激性。 |
10. 生态资料
| 一般注意事项: | 无可用资料。 |
11. 废弃处置
| 按照所在国家,省份,县市和地方的法规处置。 |
12. 运输信息
| 正确的运输名称: | 无 |
| 非危险品运输: | 这种物质被视为非危险品运输。 |
13. 法规信息
| 尚未有针对此产品作出的化学安全性评估。 |
14. 其他信息
| 这种化学品仅供受过训练的,有经验的研究人员在穿戴适当装备和授权允许的情况下进行操作处理。以上信息基于我们目前的知识被认为是正确的,但只适用于作为有经验人员的指导。请咨询您自己的安全顾问,并遵守当地和国家的安全法规。在任何其他没有被警告的情况下,并不意味着绝对没有危险存在。西域生物技术不承担任何使用这种化学品所造成的损害和责任。2023 西域生物技术版权所有。 |
|
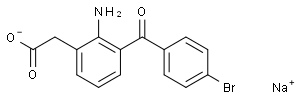
~55% 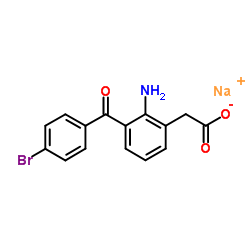
91714-93-1 |
| 文献:Walsh; Moran; Shamblee; Uwaydah; Welstead Jr.; Sancilio; Dannenburg Journal of Medicinal Chemistry, 1984 , vol. 27, # 11 p. 1379 - 1388 |
| 上游产品 1 | |
|---|---|
| 下游产品 0 | |
 有竞争力的价格
有竞争力的价格匹配竞争对手的价格
 极速物流
极速物流效率为先
 技术支持
技术支持专业经验 贴心服务
 现货库存
现货库存50000+库存


 浙公网安备 33010802013016号
浙公网安备 33010802013016号