| 产品介绍 |
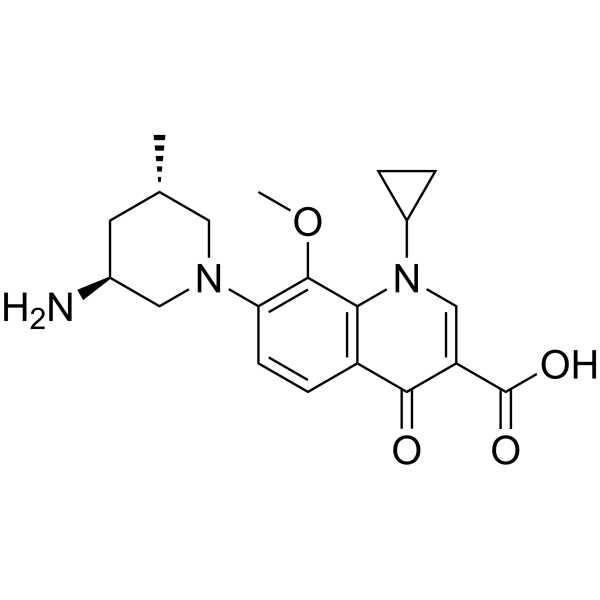
Nemonoxacin (TG-873870) 是一种口服有效的强效广谱抗生素。Nemonoxacin 对不同种类的葡萄球菌、链球菌和肠球菌、淋病奈瑟菌和流感嗜血杆菌均具有较好的抑制活性。Nemonoxacin 可用于细菌感染和社区获得性肺炎的研究。
|
|---|
| 生物活性 |
Nemonoxacin (TG-873870) is an orally active and potent broad-spectrum antibiotic. Nemonoxacin shows good inhibitory activity against different species of staphylococci, streptococci, and enterococci, Neisseria gonorrhoeae, and Haemophilus influenza. Nemonoxacin can be used in the study of bacterial infections and community-acquired pneumonia.
|
|---|
| 体外研究 |
Nemonoxacin (0-5.51 µM; 24 h) shows good antibacterial activity in vitro.
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
Cell Viability Assay
| Cell Line: |
MSSA, MRSA |
| Concentration: |
0-5.51 µM (0-2048 µg/mL) |
| Incubation Time: |
24 h |
| Result: |
Inhibited MSSA and MRSA with MIC90 values of 0.12 and 4 µg/mL. |
|
|---|
体内研究
(In Vivo) |
Nemonoxacin (p.o.; 15 min and 6 h after infection) shows potent and broad-spectrum in vivo activity against both Gram-positive (S. aureus, S. capitis, S. pneumonia and E. faecalis) and Gram-negative (E. coli) isolates.
Nemonoxacin (p.o.; 6, 12 and 24 h after infection) shows potent activities towards (2.5, 5, 10, 20 mg/kg) S. pneumonia 0613 (PRSP) and (10, 20, 40, 80 mg/kg) K. pneumonia 0607 infections in mouse pulmonary infection model.
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
CD-1 ICR mice (18-22 g; mouse systemic infection model). |
| Dosage: |
1.6-4.0 mg/kg (S. aureus and S. capitis infections), 2.4-10.0 mg/kg (S. pneumonia infections), 5.0-22.6 mg/kg (E. faecalis infections), 1.6-10.0 mg/kg (E. coli infections) |
| Administration: |
Oral administration; 15 min and 6 h after infection |
| Result: |
Showed the ED50s of 2.08, 2.59 and 2.52 mg/kg to against S. aureus ATCC 29213 (MSSA), S. aureus 0705 (MRSA) and S. capitis 0687 (levofloxacin-resistant MRSC), respectively. |
| Animal Model: |
CD-1 ICR mice (18-22 g; mouse pulmonary infection model). |
| Dosage: |
2.5, 5, 10, 20 mg/kg (S. pneumonia 0613 (PRSP)); 10, 20, 40, 80 mg/kg (K. pneumonia 0607) |
| Administration: |
Oral administration; 6, 12 and 24 h after infection |
| Result: |
Significantly decreased colony counts in vivo. |
|
| 体内研究 |
Nemonoxacin (p.o.; 15 min and 6 h after infection) shows potent and broad-spectrum in vivo activity against both Gram-positive (S. aureus, S. capitis, S. pneumonia and E. faecalis) and Gram-negative (E. coli) isolates.
Nemonoxacin (p.o.; 6, 12 and 24 h after infection) shows potent activities towards (2.5, 5, 10, 20 mg/kg) S. pneumonia 0613 (PRSP) and (10, 20, 40, 80 mg/kg) K. pneumonia 0607 infections in mouse pulmonary infection model.
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
CD-1 ICR mice (18-22 g; mouse systemic infection model). |
| Dosage: |
1.6-4.0 mg/kg (S. aureus and S. capitis infections), 2.4-10.0 mg/kg (S. pneumonia infections), 5.0-22.6 mg/kg (E. faecalis infections), 1.6-10.0 mg/kg (E. coli infections) |
| Administration: |
Oral administration; 15 min and 6 h after infection |
| Result: |
Showed the ED50s of 2.08, 2.59 and 2.52 mg/kg to against S. aureus ATCC 29213 (MSSA), S. aureus 0705 (MRSA) and S. capitis 0687 (levofloxacin-resistant MRSC), respectively. |
| Animal Model: |
CD-1 ICR mice (18-22 g; mouse pulmonary infection model). |
| Dosage: |
2.5, 5, 10, 20 mg/kg (S. pneumonia 0613 (PRSP)); 10, 20, 40, 80 mg/kg (K. pneumonia 0607) |
| Administration: |
Oral administration; 6, 12 and 24 h after infection |
| Result: |
Significantly decreased colony counts in vivo. |
|
|---|
| 体内研究 |
Nemonoxacin (p.o.; 15 min and 6 h after infection) shows potent and broad-spectrum in vivo activity against both Gram-positive (S. aureus, S. capitis, S. pneumonia and E. faecalis) and Gram-negative (E. coli) isolates.
Nemonoxacin (p.o.; 6, 12 and 24 h after infection) shows potent activities towards (2.5, 5, 10, 20 mg/kg) S. pneumonia 0613 (PRSP) and (10, 20, 40, 80 mg/kg) K. pneumonia 0607 infections in mouse pulmonary infection model.
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
CD-1 ICR mice (18-22 g; mouse systemic infection model). |
| Dosage: |
1.6-4.0 mg/kg (S. aureus and S. capitis infections), 2.4-10.0 mg/kg (S. pneumonia infections), 5.0-22.6 mg/kg (E. faecalis infections), 1.6-10.0 mg/kg (E. coli infections) |
| Administration: |
Oral administration; 15 min and 6 h after infection |
| Result: |
Showed the ED50s of 2.08, 2.59 and 2.52 mg/kg to against S. aureus ATCC 29213 (MSSA), S. aureus 0705 (MRSA) and S. capitis 0687 (levofloxacin-resistant MRSC), respectively. |
| Animal Model: |
CD-1 ICR mice (18-22 g; mouse pulmonary infection model). |
| Dosage: |
2.5, 5, 10, 20 mg/kg (S. pneumonia 0613 (PRSP)); 10, 20, 40, 80 mg/kg (K. pneumonia 0607) |
| Administration: |
Oral administration; 6, 12 and 24 h after infection |
| Result: |
Significantly decreased colony counts in vivo. |
|
|---|
| 性状 | Solid |
|---|
| 溶解性数据 | |
|---|
| 运输条件 |
Room temperature in continental US; may vary elsewhere.
|
|---|
| 储存方式 |
| Powder |
-20°C |
3 years |
|
4°C |
2 years |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
|
|---|
| 参考文献 | |
|---|






 浙公网安备 33010802013016号
浙公网安备 33010802013016号