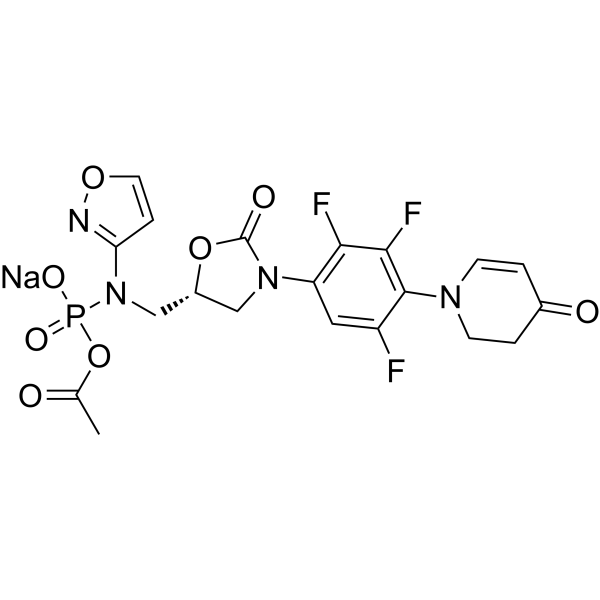
Contezolid acefosamil sodium MRX-4 sodium,99.38%
产品编号:Bellancom-19915B| CAS NO:1807365-35-0| 分子式:C20H17F3N4NaO8P| 分子量:552.33
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Contezolid acefosamil sodium MRX-4 sodium
| 产品介绍 | Contezolid acefosamil sodium (MRX-4) 是一种新型的恶唑烷酮,用于耐药革兰氏阳性菌引起的皮肤组织感染并发症 (cSSTI) 的抗生素。Contezolid acefosamil sodium (MRX-4) 可显著降低骨髓移植和单胺氧化酶抑制。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Contezolid acefosamil sodium (MRX-4), a new and orally active oxazolidinone, is an antibiotic in study for complicated skin and soft tissue infections (cSSTI) caused by resistant Gram-positive bacteria. Contezolid acefosamil sodium (MRX-4) markedly reduces potential for myelosuppression and monoamine oxidase inhibition (MAOI). | ||||||||||||||||
| 体外研究 |
Contezolid (MRX-I) is highly potent against all Grampositive clinical isolates of staphylococci, streptococci, and enterococci, including MDR organisms such as MRSA, methicilline-resistant Streptococcus epidermidis (MRSE), penicillin-resistant Streptococci (PRSP), and VRE. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Oral absorption of Contezolid (MRX-I) occurrs rapidly in mouse, rat, and dog, with peak plasma concentrations observed at 0.5−2.6 h postdose. In mouse, rat, and dog, respectively, PK parameters are determined as follows: dose-normalized Cmax/dose was 524, 1065, and 259 ng/mL/(mg/kg); dose-normalized AUC0−t/dose was 1654, 3703, and 1664 ng•h/mL/(mg/kg); T1/2 is 1, 1.5, and 3 h; and the oral bioavailability is 69%, 109%, and 37%. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 体内研究 |
Oral absorption of Contezolid (MRX-I) occurrs rapidly in mouse, rat, and dog, with peak plasma concentrations observed at 0.5−2.6 h postdose. In mouse, rat, and dog, respectively, PK parameters are determined as follows: dose-normalized Cmax/dose was 524, 1065, and 259 ng/mL/(mg/kg); dose-normalized AUC0−t/dose was 1654, 3703, and 1664 ng•h/mL/(mg/kg); T1/2 is 1, 1.5, and 3 h; and the oral bioavailability is 69%, 109%, and 37%. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : 180 mg/mL (325.89 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
-80°C, stored under nitrogen | ||||||||||||||||
| 参考文献 |
|






 浙公网安备 33010802013016号
浙公网安备 33010802013016号