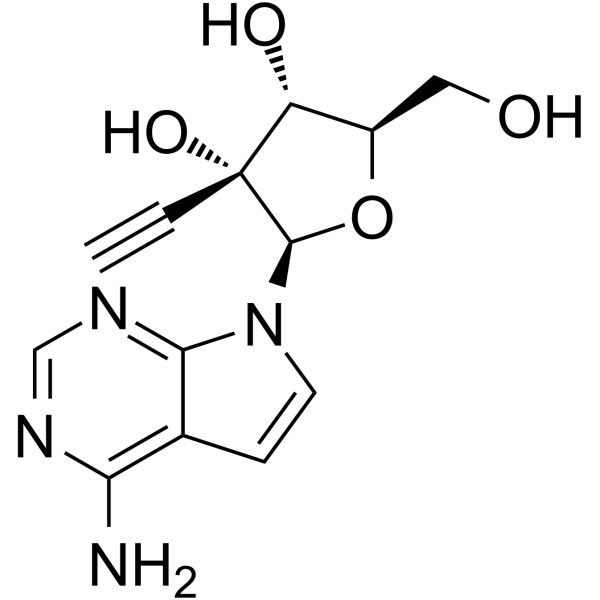
NITD008 7-Deaza-2'-C-acetylene-adenosine,98.04%
产品编号:Bellancom-12957| CAS NO:1044589-82-3| 分子式:C13H14N4O4| 分子量:290.27
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
NITD008 7-Deaza-2'-C-acetylene-adenosine
| 产品介绍 | NITD008是一种有效的和选择性的黄病毒 (flaviviruses) 抑制剂,抑制登革热病毒2型 (Dengue Virus Type 2 (DENV-2)) 的 EC50 值为0.64 μM。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | NITD008 is a potent and selective flaviviruse inhibitor which can inhibit Dengue Virus Type 2 (DENV-2) with an EC50 of 0.64 μM. | ||||||||||||||||
| 体外研究 |
NITD008 potently inhibits other, including Dengue virus (DENV), West Nile virus, yellow fever virus, and Poissan virus. NITD008 inhibits DENV-2 in a dose-responsive manner, with an EC50 value of 0.64 μM; treatment with 9 μM compound reduces viral titer by >104-fold. NITD008 also inhibits a luciferase-reporting replicon of hepatitis C virus (HCV, genotype 1b), a member from the genus Hepacivirus, with an EC50 value of 0.11 μM. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
NITD008 is orally bioavailable and has good pharmacokinetic properties. NITD008 exhibits the best pharmacokinetic parameters when formulated using 6 N of HCl (1.5 equimolar amount), 1 N of NaOH (pH adjusted to 3.5), and 100 mM citrate buffer (pH 3.5). Following i.v. injection, NITD008 has a high volume of distribution (3.71 L/kg) and a low systemic clearance (31.11 mL/min per kg), resulting in a long elimination half-life (t1/2=4.99 h). After p.o. dosing, NITD008 is rapidly absorbed (time of peak plasma concentration=0.5 h), with a maximal plasma concentration of 3 μM and bioavailability of 48%. Treatment of the mice immediately after viral infection with 1 mg/kg of NITD008 does not reduce mortality, but treatment with 3 mg/kg partially protects and treatment with ≥10 mg/kg completely protects the infected mice from death. NITD008 can suppress peak viremia, decrease cytokine elevation, and prevent death. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
NITD008 is orally bioavailable and has good pharmacokinetic properties. NITD008 exhibits the best pharmacokinetic parameters when formulated using 6 N of HCl (1.5 equimolar amount), 1 N of NaOH (pH adjusted to 3.5), and 100 mM citrate buffer (pH 3.5). Following i.v. injection, NITD008 has a high volume of distribution (3.71 L/kg) and a low systemic clearance (31.11 mL/min per kg), resulting in a long elimination half-life (t1/2=4.99 h). After p.o. dosing, NITD008 is rapidly absorbed (time of peak plasma concentration=0.5 h), with a maximal plasma concentration of 3 μM and bioavailability of 48%. Treatment of the mice immediately after viral infection with 1 mg/kg of NITD008 does not reduce mortality, but treatment with 3 mg/kg partially protects and treatment with ≥10 mg/kg completely protects the infected mice from death. NITD008 can suppress peak viremia, decrease cytokine elevation, and prevent death. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 50 mg/mL (172.25 mM) * "≥" means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |






 浙公网安备 33010802013016号
浙公网安备 33010802013016号