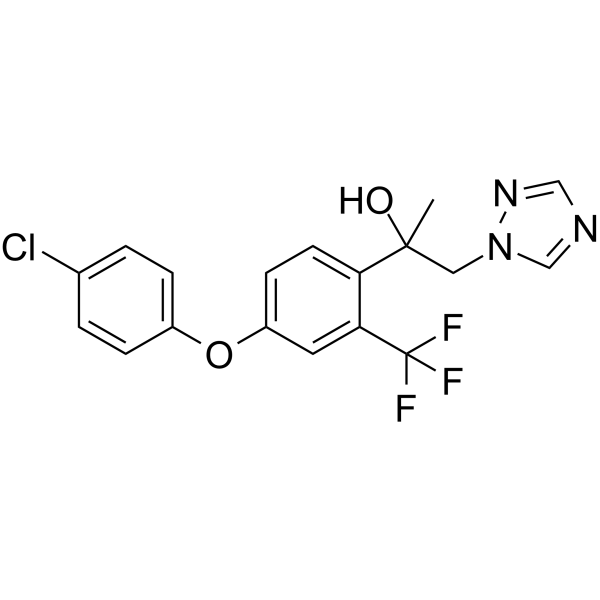
Mefentrifluconazole,99.61%
产品编号:Bellancom-136063| CAS NO:1417782-03-6| 分子式:C18H15ClF3N3O2| 分子量:397.78
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Mefentrifluconazole
| 产品介绍 | Mefentrifluconazole 是一种新型的唑类衍生物,广谱抗真菌 (antifungal agent) 剂。Mefentrifluconazole 是具有强效选择性和口服活性真菌 CYP51 的抑制剂 (Kd= 0.5 nM),但是对人类芳香化酶 (IC50=0.92 μM) 的抑制活性较小。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Mefentrifluconazole is a novel azole derivative and used as an agrochemical broad-spectrum antifungal agent. Mefentrifluconazole is a potent, selective and orally active fungal CYP51 (Kd= 0.5 nM) inhibitor, but shows less inhibitory activity on human aromatase (IC50=0.92 μM). | ||||||||||||||||
| 体外研究 | |||||||||||||||||
| 体内研究 |
Mefentrifluconazole undergoes extensive toxicity testing, including a full program of reproductive toxicity studies. Long term repeated dose toxicity and/or carcinogenicity studies have been conducted in rats, mice, and dogs. In each species, the highest dose level investigated gives rise to systemic toxicity.In the acute and repeat dose toxicity studies performed with Mefentrifluconazole. A single-dose administration to rats the LD50 is >2000 mg/kg bwt by the oral route, >5000 mg/kg bwt by the dermal route, and >5.314 mg/L by inhalation as a dust aerosol. Mefentrifluconazole is not a skin or an eye irritant, nor is it a phototoxicant in vitro.In the acute neurotoxicity study in rats, Mefentrifluconazole (oral administration; 2000 mg/kg bwt; single dose) gives rise to reduce body weight gain and transient neurobehavioral effects only on the day of treatment (unsteady gait, reduced motor activity, reduces grip strength of the forelimbs and increased distance between the hind limbs in the landing foot-splay test).In the repeated-dose toxicity studies, the liver is the target organ in each of the three species investigated. At higher dose levels in the rat (oral diets; 383/334 mg/kg/bwt/d (4000 ppm)) and the C57BL/6JRj mouse (61 mg/kg bwt/d (300 ppm)), reduces body weight gain and food consumption, alters clinical chemistry parameters, increases liver weight and is accompanied by liver cell hypertrophy, and/or liver cell necrosis. At low doses, increases liver weight is not associated with any histopathological alterations and is considered to be an adaptive change to treatment. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Mefentrifluconazole undergoes extensive toxicity testing, including a full program of reproductive toxicity studies. Long term repeated dose toxicity and/or carcinogenicity studies have been conducted in rats, mice, and dogs. In each species, the highest dose level investigated gives rise to systemic toxicity.In the acute and repeat dose toxicity studies performed with Mefentrifluconazole. A single-dose administration to rats the LD50 is >2000 mg/kg bwt by the oral route, >5000 mg/kg bwt by the dermal route, and >5.314 mg/L by inhalation as a dust aerosol. Mefentrifluconazole is not a skin or an eye irritant, nor is it a phototoxicant in vitro.In the acute neurotoxicity study in rats, Mefentrifluconazole (oral administration; 2000 mg/kg bwt; single dose) gives rise to reduce body weight gain and transient neurobehavioral effects only on the day of treatment (unsteady gait, reduced motor activity, reduces grip strength of the forelimbs and increased distance between the hind limbs in the landing foot-splay test).In the repeated-dose toxicity studies, the liver is the target organ in each of the three species investigated. At higher dose levels in the rat (oral diets; 383/334 mg/kg/bwt/d (4000 ppm)) and the C57BL/6JRj mouse (61 mg/kg bwt/d (300 ppm)), reduces body weight gain and food consumption, alters clinical chemistry parameters, increases liver weight and is accompanied by liver cell hypertrophy, and/or liver cell necrosis. At low doses, increases liver weight is not associated with any histopathological alterations and is considered to be an adaptive change to treatment. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : 100 mg/mL (251.40 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |






 浙公网安备 33010802013016号
浙公网安备 33010802013016号