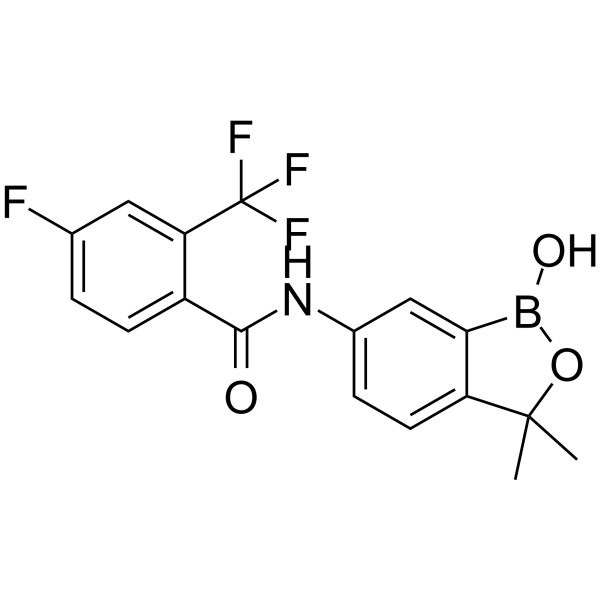
Acoziborole SCYX-7158; AN5568,99.94%
产品编号:Bellancom-19910| CAS NO:1266084-51-8| 分子式:C17H14BF4NO3| 分子量:367.10
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Acoziborole SCYX-7158; AN5568
| 产品介绍 | Acoziborole (SCYX-7158) 是一种安全有效的抗原虫剂,可用于人类非洲锥虫病 (HAT) 的研究。作用于 T. b. brucei S427 菌株,MIC 值为 0.6 µg/mL。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Acoziborole (SCYX-7158) is an effective, safe and orally active antiprotozoal agent for the research of human african trypanosomiasis (HAT). In the T. b. brucei S427 strain, the MIC value for SCYX-7158 is 0.6 µg/mL. | ||||||||||||||||
| 体外研究 |
Acoziborole is active in vitro against relevant strains of Trypanosoma brucei, including T. b. rhodesiense and T. b. gambiense.In whole cell assays, Acoziborole exhibits potent activity against representative T. b. brucei, T. b. rhodesiense and T. b. gambiense strains. IC50 values for Acoziborole are approximately 0.07 µg/mL to 0.37 µg/mL following incubation of the parasite strains with Acoziborole for 72 h. In the T. b. brucei S427 strain, the MIC value for Acoziborole is 0.6 µg/mL, approximately two times the IC50 measured for this strain. In contrast to the potent activity of Acoziborole against trypanosomes, no significant inhibition of cell proliferation is observed in an in vitro mammalian cell (L929 mouse cell line) assay at drug concentrations up to 50 µg/mL. The potential for Acoziborole to inhibit cytochrome P450 (CYP) enzymes is evaluated using P450-Glo assays for the human isoforms CYP3A4, CYP1A2, CYP2C19, CYP2C9 and CYP2D6. The IC50 values for Acoziborole in these assays are all above 10 µM. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
In uninfected mice, 4.3 mg/kg intravenous dose of Acoziborole show an apparent elimination half-life (t1/2) of 26.6 h; systemic clearance (CL) of 0.089 L/h/kg; a volume of distribution (Vdss) of 1.69 L/kg and area under the concentration-time curve (AUC0-24 h) of 48 h•μg/mL. Following an oral dose of 13.4 mg/kg, which corresponds to the lowest efficacious dose in the murine stage 2 HAT model, Acoziborole is rapidly absorbed, as a Cmax of 6.96 µg/mL is achieved in plasma at 6 h after dose, with an oral clearance (Cl/F) value of 0.163 L/h/kg, an AUC0-24 h of 82 h•μg/mL and absolute oral bioavailability of 55%. After a 26 mg/kg oral dose, which corresponds to the dose giving a 100% cure rate in the murine stage 2 HAT model, Cmax increases to 9.8 µg/mL and the AUC0-24 h is 113 h•μg/mL. In uninfected rats, following oral administration of Acoziborole at a nominal dose of 25 mg/kg (dose affording a 100% cure rate in mice), Cmax increases approximately 2 fold more than that in mice (Cmax=18.2 µg/mL) and AUC0-24 h, and hence oral clearance, improves approximately 4 fold (AUC0-24 h 291 h•μg/mL and CL/F=0.092 L/kg/h). The time to maximum concentration is similar to that in mice (tmax=8 h). Uninfected male and female cynomolgus monkeys are treated with Acoziborole at 2 mg/kg (IV) on study day 1 and 10 mg/kg (NG) on study day 8. Acoziborole exhibits excellent plasma pharmacokinetics, with CL of 0.022 L/h/kg; Vdss of 0.656 L/kg and area under the concentration-time curve 78.8 h•μg/mL, and 94.4 for AUC0-24 h and AUC0-inf, respectively, following intravenous administration. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
In uninfected mice, 4.3 mg/kg intravenous dose of Acoziborole show an apparent elimination half-life (t1/2) of 26.6 h; systemic clearance (CL) of 0.089 L/h/kg; a volume of distribution (Vdss) of 1.69 L/kg and area under the concentration-time curve (AUC0-24 h) of 48 h•μg/mL. Following an oral dose of 13.4 mg/kg, which corresponds to the lowest efficacious dose in the murine stage 2 HAT model, Acoziborole is rapidly absorbed, as a Cmax of 6.96 µg/mL is achieved in plasma at 6 h after dose, with an oral clearance (Cl/F) value of 0.163 L/h/kg, an AUC0-24 h of 82 h•μg/mL and absolute oral bioavailability of 55%. After a 26 mg/kg oral dose, which corresponds to the dose giving a 100% cure rate in the murine stage 2 HAT model, Cmax increases to 9.8 µg/mL and the AUC0-24 h is 113 h•μg/mL. In uninfected rats, following oral administration of Acoziborole at a nominal dose of 25 mg/kg (dose affording a 100% cure rate in mice), Cmax increases approximately 2 fold more than that in mice (Cmax=18.2 µg/mL) and AUC0-24 h, and hence oral clearance, improves approximately 4 fold (AUC0-24 h 291 h•μg/mL and CL/F=0.092 L/kg/h). The time to maximum concentration is similar to that in mice (tmax=8 h). Uninfected male and female cynomolgus monkeys are treated with Acoziborole at 2 mg/kg (IV) on study day 1 and 10 mg/kg (NG) on study day 8. Acoziborole exhibits excellent plasma pharmacokinetics, with CL of 0.022 L/h/kg; Vdss of 0.656 L/kg and area under the concentration-time curve 78.8 h•μg/mL, and 94.4 for AUC0-24 h and AUC0-inf, respectively, following intravenous administration. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 125 mg/mL (340.51 mM) * "≥" means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |






 浙公网安备 33010802013016号
浙公网安备 33010802013016号