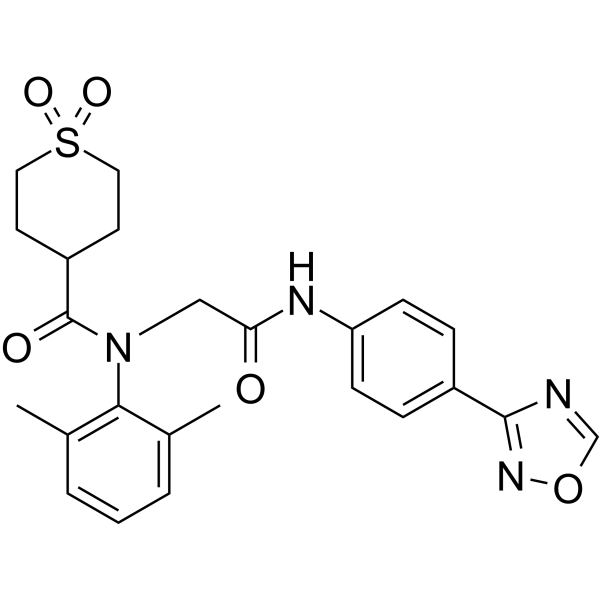
Amenamevir 阿莫奈韦; ASP2151,99.91%
产品编号:Bellancom-14809| CAS NO:841301-32-4| 分子式:C24H26N4O5S| 分子量:482.55
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Amenamevir 阿莫奈韦; ASP2151
| 产品介绍 | Amenamevir 是一种 helicase-primase 抑制剂,对 HSVs 具有强效的抗病毒活性, EC50 值为 14 ng/mL。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Amenamevir is a helicase-primase inhibitor which has potent antiviral activity against HSVs with an EC50 of 14 ng/mL. | ||||||||||||||||
| 体外研究 |
Amenamevir (ASP2151) inhibits the replication of the HSV strains isolated in Japan and the United States as well as the laboratory-stocked strains. The mean EC50s of Amenamevir against HSV-1 and HSV-2 are 14 (range, 7.7 to 20) and 30 ng/mL (range, 15 to 58), respectively, whereas those of acyclovir (ACV) are 29 (range, 18 to 38) and 71 ng/mL (range, 45 to 95), respectively. The EC50s of Amenamevir against HSV strains are significantly lower than those of ACV. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Amenamevir (ASP2151) administration accelerates the reduction in virus titer in a dose-dependent manner in the range of 3 to 30 mg/kg/day. Amenamevir treatment decreases both lesion scores and HSV-1 titers in a dose-dependent manner, irrespective of the dosing interval. Based on the correlation curves, the PK parameters at which HSV-1 growth is completely suppressed by oral administration of Amenamevir are estimated to be 10,000 ng/mL or higher for the maximum concentration of drug in serum (Cmax), 60 μg • h/ml or higher for concentration-time curve over 24 h (AUC24h), and 21 to 24 h for T>100 . The mean concentration of Amenamevir in plasma at 5 days postinfection increases in a dose-dependent manner, with doses of 3 mg Amenamevir/g or higher significantly reducing the intradermal HSV-1 titer. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Amenamevir (ASP2151) administration accelerates the reduction in virus titer in a dose-dependent manner in the range of 3 to 30 mg/kg/day. Amenamevir treatment decreases both lesion scores and HSV-1 titers in a dose-dependent manner, irrespective of the dosing interval. Based on the correlation curves, the PK parameters at which HSV-1 growth is completely suppressed by oral administration of Amenamevir are estimated to be 10,000 ng/mL or higher for the maximum concentration of drug in serum (Cmax), 60 μg • h/ml or higher for concentration-time curve over 24 h (AUC24h), and 21 to 24 h for T>100 . The mean concentration of Amenamevir in plasma at 5 days postinfection increases in a dose-dependent manner, with doses of 3 mg Amenamevir/g or higher significantly reducing the intradermal HSV-1 titer. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 50 mg/mL (103.62 mM) * "≥" means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |






 浙公网安备 33010802013016号
浙公网安备 33010802013016号