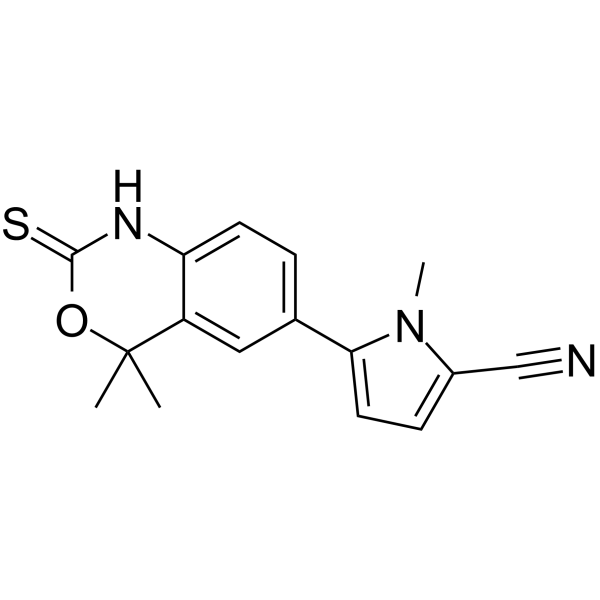
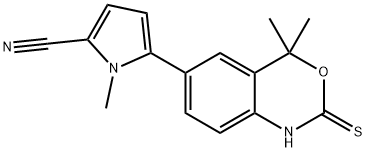
Tanaproget NSP-989,99.87%
产品编号:Bellancom-15606| CAS NO:304853-42-7| 分子式:C16H15N3OS| 分子量:297.37
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Tanaproget NSP-989
| 产品介绍 | Tanaproget (NSP-989) 是新型非甾体孕酮受体激动剂。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Tanaproget (NSP-989) is a novel nonsteroidal progesterone receptor agonist which can bind to the PR from various species with a higher relative affinity than reference steroidal progestins. IC50 value: 0.1 nM (EC50, induce alkaline phosphatase activity) Target: progesterone receptor Tanaproget represents a potential first-in-class nonsteroidal PR agonist for contraception with improved safety and side effect profiles versus currently available steroidal oral contraceptives. in vitro: In T47D cells, TNPR induces alkaline phosphatase activity with an EC(50) value of 0.1 nm, comparable with potent steroidal progestins such as medroxyprogesterone acetate (MPA) and trimegestone (TMG), albeit with a reduced efficacy ( approximately 60%). In a mammalian two-hybrid assay to measure PR agonist-induced interaction between steroid receptor co-activator-1 and PR, TNPR showed similar potency (EC(50) value of 0.02 nm) and efficacy to MPA and TMG . in vivo: TNPR effectively down-regulated MMP expression in vitro and induced significant reduction of lesions in mice with disease established by tissues from endometriosis patients . The maximum concentration (C(max)) of tanaproget occurred approximately 2 to 3 h after administration. The elimination half-life (t(1/2)) ranged from 12 to 30 h, and the oral clearance was approximately 70 L/h. The pharmacokinetics of tanaproget was not noticeably altered with a high-fat meal . Toxicity: All doses of tanaproget decreased cervical mucus scores (using a modified Insler method), indicating poor production and poor quality of cervical mucus. The most frequent treatment-emergent adverse events were vaginal bleeding/spotting, abdominal cramping and vomiting; their incidence was not dose related and most events were mild . | ||||||||||||||||
| 体外研究 | |||||||||||||||||
| 体内研究 | |||||||||||||||||
| 体内研究 | |||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 50 mg/mL (168.14 mM) * "≥" means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
*以上所有助溶剂都可在 西域 网站选购。
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |
|






 浙公网安备 33010802013016号
浙公网安备 33010802013016号