MK-0354,99.21%
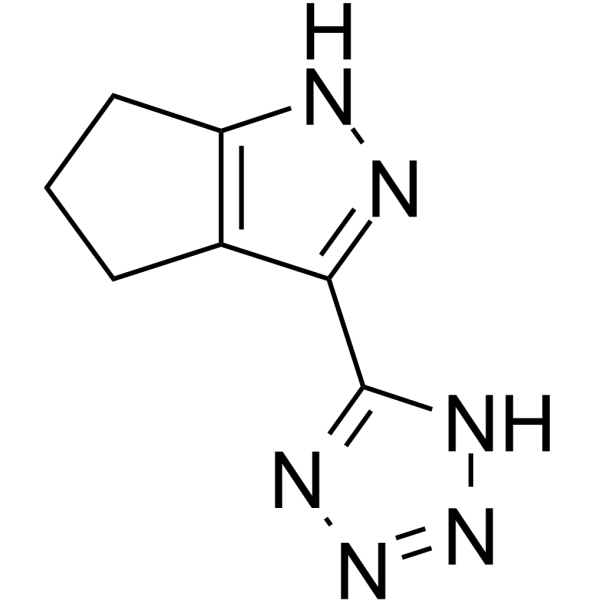
产品编号:Bellancom-13008| CAS NO:851776-28-8| 分子式:C7H8N6| 分子量:176.18
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
MK-0354
| 产品介绍 | MK-0354是特异性的 GPR109a 受体激动剂, 作用于hGPR109a/mGPR109a, EC50分别为1.65 μM 和1.08 μM, 对GPR109b没有作用效果。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | MK-0354 is a partial agonist of GPR109a receptor, for hGPR109a/ mGPR109a with EC50 of 1.65/1.08 μM, showed no activation of GPR109b. IC50 value: 1.65 μM (EC50, for hGPR109a), 1.08 μM (EC50, for mGPR109a) Target: GPR109a in vitro: MK-0354 demonstrated clear and statistically significant partial agonism in the cAMP assays for both the mouse and human receptors with efficacy approximately 60-70% of that of either nicotinic acid or β-hydroxy butyrate, a putative physiologically relevant ligand for hGPR109a, in the same assay platform. In addition, MK-0354 showed no activation of GPR109b in the cAMP assay at any concentration up to 100 μM. Following these interesting observations, we then prepared a number of other 5,5-fused pyrazoles analogous to those that showed receptor activity in our earlier studies. MK-0354 appeared to be somewhat unique among the members of the pyrazole tetrazole series in having reasonable receptor activity. in vivo: MK-0354 retained the plasma free fatty acid lowering effects in mice associated with GPR109a agonism, but did not induce vasodilation at the maximum feasible dose. Moreover, preadministration of MK-0354 blocked the flushing effect induced by nicotinic acid but not that induced by PGD2. This profile made MK-0354 a suitable candidate for further study for the treatment of dyslipidemia. MK-0354 is a GPR109A partial agonist that activates the antilipolytic pathway in adipocytes. The single-dose and multiple-dose pharmacokinetics and pharmacodynamics, as well as tolerability, of MK-0354 were examined in two Phase I studies conducted in healthy male volunteers. The lipid efficacy of MK-0354 was assessed in a Phase II study conducted in male and female patients with dyslipidemia. | ||||||||||||||||
| 体外研究 | |||||||||||||||||
| 体内研究 | |||||||||||||||||
| 体内研究 | |||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 36 mg/mL (204.34 mM) * "≥" means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |
|






 浙公网安备 33010802013016号
浙公网安备 33010802013016号