EPZ015666 GSK3235025,99.13%
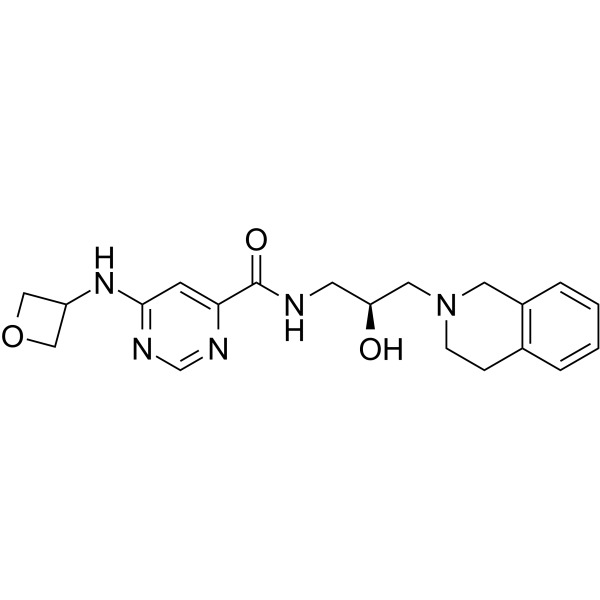
产品编号:Bellancom-12727| CAS NO:1616391-65-1| 分子式:C20H25N5O3| 分子量:383.44
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
EPZ015666 GSK3235025
| 产品介绍 | EPZ015666 (GSK3235025) 是有口服活性的 PRMT5抑制剂,IC50 为 22 nM。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | EPZ015666 (GSK3235025) is an orally available inhibitor of PRMT5 with an IC50 of 22 nM. | ||||||||||||||||
| 体外研究 |
Treatment of MCL cell lines with EPZ015666 (GSK3235025) leads to inhibition of SmD3 methylation and cell death, with IC50 values in the nanomolar range. EPZ015666 (GSK3235025), a potent peptide-competitive and SAM-cooperative inhibitor with >10,000-fold specificity against PRMT5 relative to other methyltransferases. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
EPZ015666 (GSK3235025) is orally bioavailable and amenable to in vivo studies. We performed 21-d efficacy studies in severe combined immunodeficiency (SCID) mice bearing subcutaneous Z-138 and Maver-1 xenografts, with twice-daily (BID) oral dosing on four dose groups: 25, 50, 100 and 200 mg per kilogram of body weight (mg/kg). After 21 d of continuous dosing, animals are euthanized, and blood and tissues are analyzed to determine the relationship between methyl-mark pharmacodynamics and tumor-growth inhibition (TGI). EPZ015666 (GSK3235025) showed dose-dependent exposure and TGI after 21 d in both MCL models. Tumors in all EPZ015666 (GSK3235025) dose groups measured on day 21 showed statistically significant differences in weight, volume and tumor growth compared to vehicle-treated tumors. Dosing at 200 mg/kg BID induced tumor stasis in Z-138 cells, with >93% TGI after 21 d, whereas Maver-1 cells showed >70% TGI. Additionally, a third MCL xenograft is tested using the Granta-519 cell line, a fast-growing model that reached endpoint on day 18 and showed dose-dependent efficacy with 45% TGI in the 200 mg/kg group. EPZ015666 (GSK3235025) is well tolerated in all three models, with minimal bodyweight loss in the 200 mg/kg dose group and no other clinical observations. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
EPZ015666 (GSK3235025) is orally bioavailable and amenable to in vivo studies. We performed 21-d efficacy studies in severe combined immunodeficiency (SCID) mice bearing subcutaneous Z-138 and Maver-1 xenografts, with twice-daily (BID) oral dosing on four dose groups: 25, 50, 100 and 200 mg per kilogram of body weight (mg/kg). After 21 d of continuous dosing, animals are euthanized, and blood and tissues are analyzed to determine the relationship between methyl-mark pharmacodynamics and tumor-growth inhibition (TGI). EPZ015666 (GSK3235025) showed dose-dependent exposure and TGI after 21 d in both MCL models. Tumors in all EPZ015666 (GSK3235025) dose groups measured on day 21 showed statistically significant differences in weight, volume and tumor growth compared to vehicle-treated tumors. Dosing at 200 mg/kg BID induced tumor stasis in Z-138 cells, with >93% TGI after 21 d, whereas Maver-1 cells showed >70% TGI. Additionally, a third MCL xenograft is tested using the Granta-519 cell line, a fast-growing model that reached endpoint on day 18 and showed dose-dependent efficacy with 45% TGI in the 200 mg/kg group. EPZ015666 (GSK3235025) is well tolerated in all three models, with minimal bodyweight loss in the 200 mg/kg dose group and no other clinical observations. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : 100 mg/mL (260.80 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |
|






 浙公网安备 33010802013016号
浙公网安备 33010802013016号