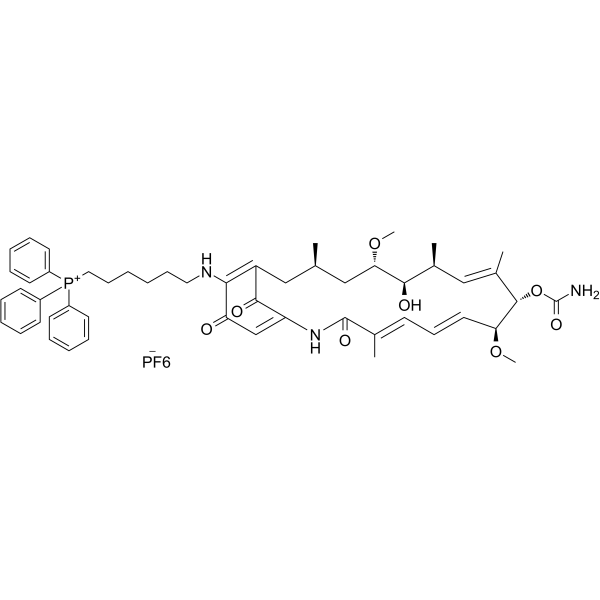
Gamitrinib TPP hexafluorophosphate,98.16%
产品编号:Bellancom-102007A| CAS NO:1131626-47-5| 分子式:C52H65F6N3O8P2| 分子量:1036.03
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
Gamitrinib TPP hexafluorophosphate
| 产品介绍 | Gamitrinib TPP hexafluorophosphate 是一种 Gamitrinib (GA) 线粒体基质的抑制剂。Gamitrinib TPP hexafluorophosphate 是一种靶向线粒体的 HSP90 抑制剂。具有抗癌活性。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Gamitrinib TPP hexafluorophosphate is a Gamitrinib (GA) mitochondrial matrix inhibitor. Gamitrinib TPP hexafluorophosphate is a mitochondrial targeted HSP90 inhibitor with anti-cancer activity. | ||||||||||||||||
| 体外研究 |
Gamitrinib TPP (GamitrinibTPP, G-TPP), a small molecule that combines the Hsp90 ATPase inhibitory module of 17-allylamino geldanamycin (17-AAG) with the mitochondrial-targeting moiety of triphenylphosphonium. Gamitrinib TPP is selectively delivered to mitochondria and does not affect Hsp90 homeostasis outside the organelle. Within a 16-hour exposure, concentrations of Gamitrinib TPP of 15-20 μM indistinguishably kill patient-derived and cultured glioblastoma cell lines. This cell death response has the hallmarks of mitochondrial apoptosis, with loss of organelle inner membrane potential, release of cytochrome c in the cytosol, activation of initiator caspase-9 and effector caspase-3 and caspase-7, and cellular reactivity for annexin V. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Whether the combination of TRAIL plus Gamitrinib TPP (GamitrinibTPP, G-TPP) has activity against glioblastoma in vivo is studied. Luciferase-expressing U87 glioblastoma cells implanted in the right cerebral striatum of immunocompromised mice give rise to rapidly growing tumors by bioluminescence imaging, and treatment of these mice with vehicle, stereotactic delivery of TRAIL, or systemic administration of suboptimal concentrations of Gamitrinib TPP does not affect tumor growth in vivo. Similarly, systemic monotherapy with Gamitrinib TPP at concentrations (20 mg/kg as daily i.p. injections) that inhibit subcutaneous xenograft tumor growth in mice has no effect on orthotopic glioblastoma growth. In contrast, 2 cycles of intracranial TRAIL combined with systemic Gamitrinib TPP suppresses the growth of established glioblastomas, with no significant animal weight loss throughout treatment. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Whether the combination of TRAIL plus Gamitrinib TPP (GamitrinibTPP, G-TPP) has activity against glioblastoma in vivo is studied. Luciferase-expressing U87 glioblastoma cells implanted in the right cerebral striatum of immunocompromised mice give rise to rapidly growing tumors by bioluminescence imaging, and treatment of these mice with vehicle, stereotactic delivery of TRAIL, or systemic administration of suboptimal concentrations of Gamitrinib TPP does not affect tumor growth in vivo. Similarly, systemic monotherapy with Gamitrinib TPP at concentrations (20 mg/kg as daily i.p. injections) that inhibit subcutaneous xenograft tumor growth in mice has no effect on orthotopic glioblastoma growth. In contrast, 2 cycles of intracranial TRAIL combined with systemic Gamitrinib TPP suppresses the growth of established glioblastomas, with no significant animal weight loss throughout treatment. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : 50 mg/mL (48.26 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |






 浙公网安备 33010802013016号
浙公网安备 33010802013016号