碘化锗(II), 99.9% trace metals basis,Germanium(II) iodide
产品编号:西域试剂-WR365040| CAS NO:13573-08-5| MDL NO:MFCD00135537| 分子式:GeI2| 分子量:326.45
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
| 英文名称 | Germanium(II) iodide |
|---|---|
| CAS编号 | 13573-08-5 |
| 产品熔点 | 460°C |
| 产品沸点 | 545°C |
| 产品密度 | 5.37 g/mL at 25 °C(lit.) |
| 精确质量 | 329.74600 |
| LogP | 1.61560 |
| 外观性状 | 黄色至金色片或粉末 |
| 稳定性 | 常温常压下稳定 避免的物料 水分/潮湿 氧化物 光 .遇湿空气即水解,在干燥空气中稳定。在氨溶液中就生成亚胺锗。二碘化锗稍溶于冷的氢碘酸,加热就非常易溶,所以能够重结晶。在真空中于240℃升华。二碘化锗可用作有机锗化物的合成原料。 |
| 储存条件 | 常温密闭,阴凉通风干燥 |
相关文档
化学品安全说明书(MSDS)
下载MSDS质检证书(COA)
相关产品
| 符号 |
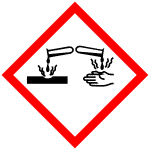
GHS05 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H314 |
| 警示性声明 | P280-P305 + P351 + P338-P310 |
| 个人防护装备 | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| 危害码 (欧洲) | C: Corrosive; |
| 风险声明 (欧洲) | R34 |
| 安全声明 (欧洲) | S23-S26-S27-S36/37/39-S45 |
| 危险品运输编码 | UN 3260 8/PG 2 |
| WGK德国 | 3 |
| 包装等级 | II |
| 危险类别 | 8 |
|
Section 1: Product Identification Chemical Name:Germanium (II) iodide (99.99+%-Ge) PURATREM CAS Registry Number:13573-08-5 Formula:GeI2 EINECS Number:236-998-1 Chemical Family:metal halide Synonym:Germanium diiodide
Section 2: Composition and Information on Ingredients IngredientCAS NumberPercentACGIH (TWA)OSHA (PEL) Title Compound13573-08-5100%no datano data Section 3: Hazards Identification Severe irritant to skin, eyes and respiratory tract. Material hydrolyzes in contact with moisture releasing Emergency Overview: hydroiodic acid and under some conditions corrosive hydrogen iodide vapors. Primary Routes of Exposure:Ingestion, inhalation, skin, eyes Eye Contact:Causes severe irritation to the eyes. Skin Contact:Causes severe irritation of the skin with redness, pain, itching, pimples, boils, hives and blisters. Forms hydroiodic acid in the presence of moisture. Inhalation of vapors leads to irritation of the respiratory Inhalation: tract. Ingestion:Severe irritation of the Gastrointestinal system may occur. Acute Health Affects:Irritating to skin, eyes and respiratory tract. May cause dyspnea (breathing difficulty), and pulmonary edema. Chronic ingestion of iodides may produce iodism. Symptoms include skin rash, running nose, headache and Chronic Health Affects: irritation of mucous membranes. NTP:No IARC:No OSHA:No SECTION 4: First Aid Measures Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need Eye Exposure: assistance in keeping their eye lids open. Get immediate medical attention. Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if Skin Exposure: irritation persists. Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty Inhalation: in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance. Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce Ingestion: vomiting only if directed by medical personnel. SECTION 5: Fire Fighting Measures Flash Point:not applicable Autoignition Temperature:none Explosion Limits:none Extinguishing Medium:none required If involved in a fire, fire fighters should be equipped with a NIOSH approved positive pressure self-contained Special Fire Fighting Procedures: breathing apparatus and full protective clothing. Hazardous Combustion andIf involved in a fire this material may emit corrosive hydrogen fluoride fumes. Decomposion Products: Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards. SECTION 6: Accidental Release Measures To avoid raising dust, small spills can be mixed with powdered sodium bicarbonate, lime, or calcium carbonate Spill and Leak Procedures: and swept up. SECTION 7: Handling and Storage Store material in a tightly sealed bottle away from moisture. If possible, handle material in an efficient fume Handling and Storage: hood. Prolonged exposure to the atmosphere may lead to degradation of the product. SECTION 8: Exposure Controls and Personal Protection Eye Protection:Always wear approved safety glasses when handling a chemical substance in the laboratory. Skin Protection:Wear appropriate chemical resistant gloves and protective clothing. Ventilation:If possible, handle the material in an efficient fume hood. If in form of fine dust and ventilation is not available a respirator should be worn. The use of respirators Respirator: requires a Respirator Protection Program to be in compliance with 29 CFR 1910.134. Ventilation:If possible, handle the material in an efficient fume hood. Additional Protection:No additional protection required. SECTION 9: Physical and Chemical Properties Color and Form:-10 mesh yellow pwdr. Molecular Weight:326.4 Melting Point:dec. Boiling Point:no data Vapor Pressure:no data Specific Gravity:5.73 Odor:not determined Solubility in Water:Decomposes in water SECTION 10: Stability and Reactivity Stability:moisture sensitive Hazardous Polymerization:no hazardous polymerization Conditions to Avoid:contact with moisture Incompatibility:Oxidizing agents, halogens and active metals Decomposition Products:with moisture: hydroiodic acid and germanium oxides SECTION 11: Toxicological Information RTECS Data:No information available in the RTECS files. Carcinogenic Effects:no data Mutagenic Effects:no data Tetratogenic Effects:no data SECTION 12: Ecological Information Ecological Information:No information available SECTION 13: Disposal Considerations Disposal:Dispose of according to local, state and federal regulations. SECTION 14: Transportation Shipping Name (CFR):Non-hazardous Hazard Class (CFR):NA Additional Hazard Class (CFR):NA Packaging Group (CFR):NA UN ID Number (CFR):NA Shipping Name (IATA):Non-hazardous Hazard Class (IATA):NA Additional Hazard Class (IATA):NA Packaging Group (IATA):NA UN ID Number (IATA):NA SECTION 15: Regulatory Information TSCA:Listed in the TSCA inventory. SARA (Title 313):Title compound not listed Second Ingredient:none SECTION 16 - ADDITIONAL INFORMATION N/A |
|
~% 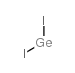
13573-08-5 |
| 文献:Journal of Chemical Physics, , vol. 91, # 5 p. 2834 - 2839 |








 浙公网安备 33010802013016号
浙公网安备 33010802013016号