Conoidin A,98.03%
产品编号:Bellancom-116090| CAS NO:18080-67-6| 分子式:C10H8Br2N2O2| 分子量:347.99
Conoidin A 是刚地弓形虫过氧化物酶 II (TgPrxII) 的细胞通透性抑制剂,有杀线虫的特性。Conoidin A 共价结合 TgPrxII 的过氧化物催化位点 Cys47,不可逆地抑制其过氧化物活性,IC50 为 23 µM。Conoidin A 也抑制哺乳动物 PrxI 和 PrxII (但不抑制 PrxIII) 的氧化。Conoidin A 具有抗氧化、神经保护作用,并且可用于缺血性心脏病的研究。
本网站销售的所有产品仅用于工业应用或者科学研究等非医疗目的,不可用于人类或动物的临床诊断或者治疗,非药用,非食用,
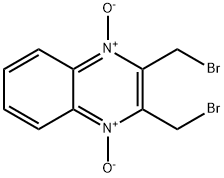
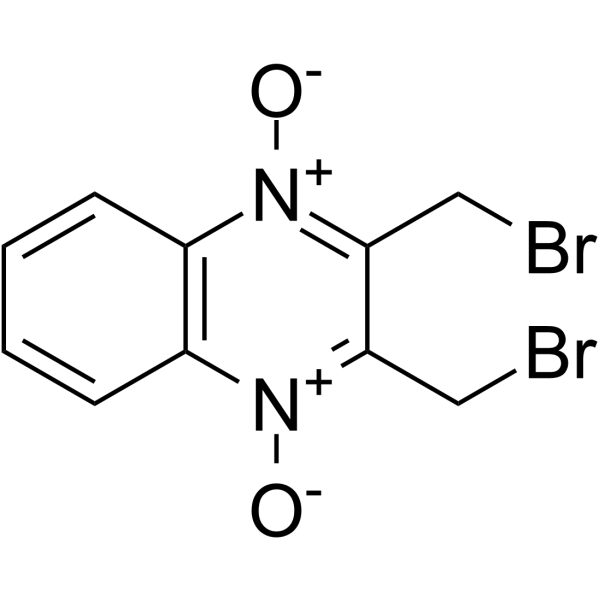
Conoidin A
| 产品介绍 | Conoidin A 是刚地弓形虫过氧化物酶 II (TgPrxII) 的细胞通透性抑制剂,有杀线虫的特性。Conoidin A 共价结合 TgPrxII 的过氧化物催化位点 Cys47,不可逆地抑制其过氧化物活性,IC50 为 23 µM。Conoidin A 也抑制哺乳动物 PrxI 和 PrxII (但不抑制 PrxIII) 的氧化。Conoidin A 具有抗氧化、神经保护作用,并且可用于缺血性心脏病的研究。 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 生物活性 | Conoidin A is a cell permeable inhibitor of T. gondii enzyme peroxiredoxin II (TgPrxII) with nematicidal properties. Conoidin A covalently binds to the peroxidatic Cys47 of TgPrxII, irreversibly inhibiting its hyperperoxidation activity with an IC50 of 23 µM. Conoidin A also inhibits hyperoxidation of mammalian PrxI and PrxII (but not PrxIII). Conoidin A has antioxidant, neuroprotective effects and can be used for the research of ischaemic heart disease. | ||||||||||||||||
| 体外研究 |
Peroxiredoxins are a widely conserved family of enzymes that function in antioxidant defense and signal transduction. And the changes in PrxII expression are associated with a variety of human diseases, including cancer.Conoidin A binds to the peroxidatic cysteine of TgPrxII, inhibiting its enzymatic activity in vitro. Conoidin A also shown to alkylate or crosslink catalytic cysteines of wild type AcePrx-1 in Ancylostoma ceylanicum and human PrxII and PrxIV with similar efficiency. But it is ineffective to mitochondrial hPrxIII.Conoidin A (5 µM) can inhibit the glucose oxidase-mediated hyperoxidation of mammalian peroxiredoxin I and II. 西域 has not independently confirmed the accuracy of these methods. They are for reference only. | ||||||||||||||||
| 体内研究 |
Conoidin A (intraperitoneal injection; 5 mg/kg; for three successive days before MI/R injury) blocks the effect of Luteolin (HY-N0162) on the ST‐segment elevation. Furthermore, an increase in the infarct size presented of the MI/R group can be reduced by Luteolin. But pre‐treatment with conoidin A abolishs the effect of Luteolin. Pre‐treatment with conoidin A also prevents Luteolin-reduced activities of CK‐MB, AST and LDH in vivo. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 体内研究 |
Conoidin A (intraperitoneal injection; 5 mg/kg; for three successive days before MI/R injury) blocks the effect of Luteolin (HY-N0162) on the ST‐segment elevation. Furthermore, an increase in the infarct size presented of the MI/R group can be reduced by Luteolin. But pre‐treatment with conoidin A abolishs the effect of Luteolin. Pre‐treatment with conoidin A also prevents Luteolin-reduced activities of CK‐MB, AST and LDH in vivo. 西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| ||||||||||||||||
| 性状 | Solid | ||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : 14.29 mg/mL (41.06 mM; Need ultrasonic) 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
| ||||||||||||||||
| 运输条件 | Room temperature in continental US; may vary elsewhere. | ||||||||||||||||
| 储存方式 |
| ||||||||||||||||
| 参考文献 |
| ||||||||||||||||
| 海关编码 | 2933990090 |
|---|
|
~89% 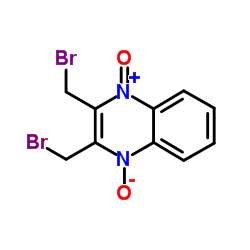
18080-67-6 |
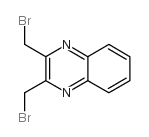
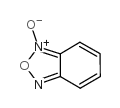
| 文献:Carmeli, Mira; Rozen, Shlomo Journal of Organic Chemistry, 2006 , vol. 71, # 15 p. 5761 - 5765 |
|
~65% 
18080-67-6 |
| 文献:Pearson, Russell J.; Evans, Kathryn M.; Slawin, Alexandra M. Z.; Philp, Douglas; Westwood, Nicholas J. Journal of Organic Chemistry, 2005 , vol. 70, # 13 p. 5055 - 5061 |
|
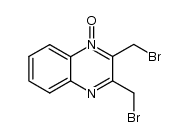
~82% 
18080-67-6 |
| 文献:Haddadin, Makhluf J.; Samaha, Mona S.; Hajj-Ubayd, Antoun B. Heterocycles, 1992 , vol. 33, # 2 p. 541 - 544 |
|
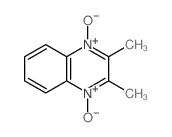
~% 
18080-67-6 |
| 文献:Pearson, Russell J.; Evans, Kathryn M.; Slawin, Alexandra M. Z.; Philp, Douglas; Westwood, Nicholas J. Journal of Organic Chemistry, 2005 , vol. 70, # 13 p. 5055 - 5061 |
|
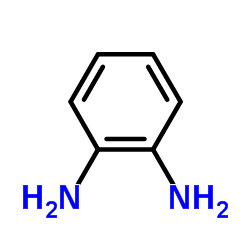
~% 
18080-67-6 |
| 文献:Haddadin, Makhluf J.; Samaha, Mona S.; Hajj-Ubayd, Antoun B. Heterocycles, 1992 , vol. 33, # 2 p. 541 - 544 |
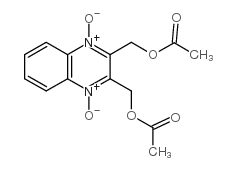
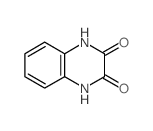
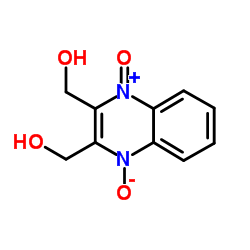
| 上游产品 4 | |
|---|---|
| 下游产品 3 | |
 有竞争力的价格
有竞争力的价格匹配竞争对手的价格
 极速物流
极速物流效率为先
 技术支持
技术支持专业经验 贴心服务
 现货库存
现货库存50000+库存


 浙公网安备 33010802013016号
浙公网安备 33010802013016号