| 产品介绍 |
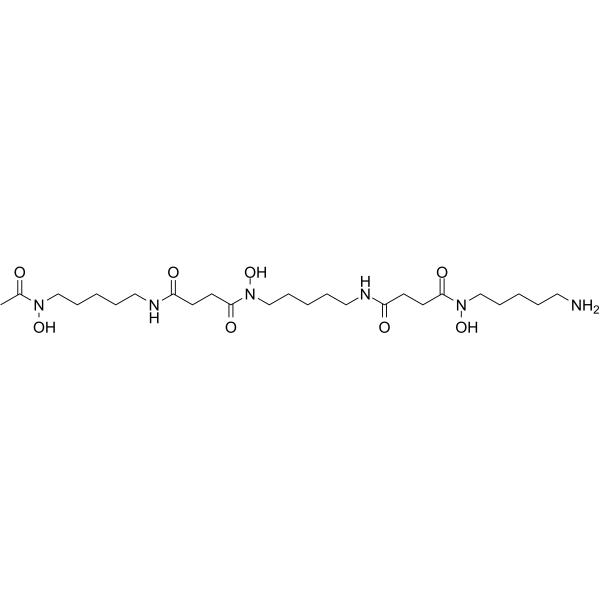
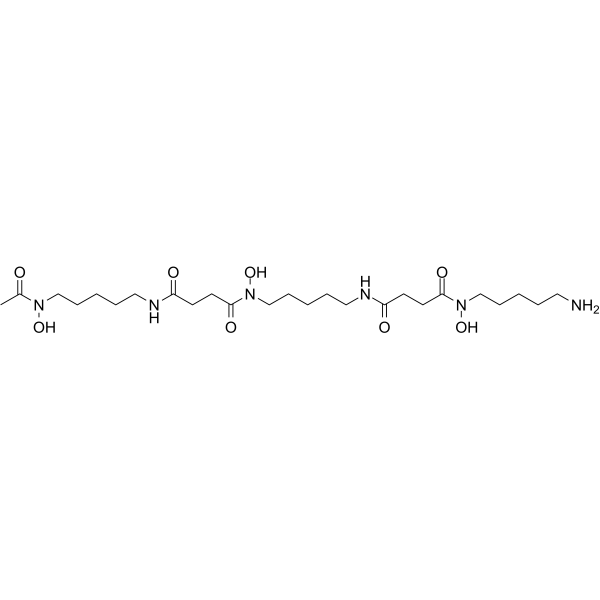
Deferoxamine (Deferoxamine B) 是一种铁螯合剂 (结合 Fe(III) 和许多其他金属阳离子),被广泛用于减少铁在组织中的积累和沉积。Deferoxamine 具有较好的抗氧化活性,可上调 HIF-1α 水平。Deferoxamine 还具有抗增殖活性,能诱导癌细胞凋亡和自噬。Deferoxamine 可用于糖尿病、神经退行性疾病以及抗癌和抗 COVID-19 的研究。
|
|---|
| 生物活性 |
Deferoxamine (Deferoxamine B) is an iron chelator (binds to Fe(III) and many other metal cations), is widely used to reduce iron accumulation and deposition in tissues. Deferoxamine upregulates HIF-1α levels with good antioxidant activity. Deferoxamine also shows anti-proliferative activity, can induce apoptosis and autophagy in cancer cells. Deferoxamine can be used in studies of diabetes, neurodegenerative diseases as well as anti-cancer and anti-COVID-19[4][5].
|
|---|
| 体外研究 |
Deferoxamine (1 mM; 16 h or 4 weeks) improves HIF-1α function under hypoxic and hyperglycemic conditions and decreases ROS in MEFs cells.
Deferoxamine (100 µM; 24 h) increases InsR expression and activity and also induces an increase in p-Akt/total Akt/PKB levels.
Deferoxamine (5, 10, 25, 50, 100 µM; 7 or 9 days) inhibits the proliferation of tumor-associated MSCs and bone marrow MSCs.
Deferoxamine (5, 10, 25, 50, 100 µM; 7 days) induces apoptosis of MSCs.
Deferoxamine (10 µM ; 3 days) influencs the expression of adhesion proteins on MSCs.
Deferoxamine (100 µM; 24 h) induces autophagy mediated by the level of HIF-1α in SH-SY5Y cells[4].
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
Western Blot Analysis
| Cell Line: |
MEFs cells |
| Concentration: |
1 mM |
| Incubation Time: |
16 h (hypoxia condition); 4 weeks (hyperglycemic conditions) |
| Result: |
Significantly attenuated the hyperglycemia-associated increase in ROS levels under hypoxic high glucose conditions.
Notably increased normoxic HIF transactivation in MEFs under both high glucose and normal glucose conditions.
|
Western Blot Analysis
| Cell Line: |
HepG2 cells |
| Concentration: |
100 µM |
| Incubation Time: |
24 h |
| Result: |
Showed a twofold increase of InsR mRNA levels in cells.
Increased by twofold InsR binding activity at the half-maximal concentration of 1.1 nM.
|
Cell Proliferation Assay
| Cell Line: |
TAMSCs and BMMSCs (all isolated from Male C57BL/6J mice (8 week-old; EG-7 induced tumor model)) |
| Concentration: |
5, 10, 25, 50, 100 µM |
| Incubation Time: |
7 days (TAMSCs); 9 days (BMMSCs). |
| Result: |
Inhibited the growth of TAMSCs and BMMSCs, and most cells are died at day 7 or 9 when exposed to 50 and 100 µM dose. |
Apoptosis Analysis
| Cell Line: |
TAMSCs, BMMSCs |
| Concentration: |
5, 10, 25, 50, 100 µM |
| Incubation Time: |
7 days |
| Result: |
Exhibited proapoptotic effect on TAMSCs and BMMSCs cells. |
Western Blot Analysis
| Cell Line: |
TAMSCs, BMMSCs |
| Concentration: |
10 µM |
| Incubation Time: |
3 days |
| Result: |
Remarkably decreased VCAM-1 expression in both TAMSCs and BMMSCs. |
Cell Autophagy Assay[4]
| Cell Line: |
SH-SY5Y cells |
| Concentration: |
100 µM |
| Incubation Time: |
24 h |
| Result: |
Increased the ratio of LC3-II/I, an indicator of autophagy, which effects were blocked when autophagy-related gene Beclin 1 was suppressed by Beclin 1 siRNA transfection.
Caused a time and dose-dependent increase of HIF-1a, accompanied by the induction of autophagy.
|
|
|---|
体内研究
(In Vivo) |
Deferoxamine (560.68 mg/per; drip-on; once daily for 21 days) enhances wound healing and increases neovascularization in aged or diabetic mice.
Deferoxamine (200 mg/kg; i.p.; daily for 2 weeks) results in HIF-1α stabilization and increases glucose uptake, hepatic InsR expression, and signaling in vivo.
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
Aged (21-month-old) and diabetic (12-week-old) C57BL/6J mice (excisional wound model). |
| Dosage: |
560.68 mg/per (10 uL of 1 mM) |
| Administration: |
Drip-on; once daily for 21 days. |
| Result: |
Displayed significantly accelerated healing and increased neovascularization in both aged and diabetic mice model. |
| Animal Model: |
Male Sprague-Dawley rats (180-200 g). |
| Dosage: |
200 mg/kg |
| Administration: |
Intraperitoneal injection; daily for 2 weeks. |
| Result: |
Significantly increased hepatic HIF-1α protein levels, InsR protein levels, as well as Akt/PKB and activated Akt/PKB were significantly higher in the liver. |
|
| 体内研究 |
Deferoxamine (560.68 mg/per; drip-on; once daily for 21 days) enhances wound healing and increases neovascularization in aged or diabetic mice.
Deferoxamine (200 mg/kg; i.p.; daily for 2 weeks) results in HIF-1α stabilization and increases glucose uptake, hepatic InsR expression, and signaling in vivo.
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
Aged (21-month-old) and diabetic (12-week-old) C57BL/6J mice (excisional wound model). |
| Dosage: |
560.68 mg/per (10 uL of 1 mM) |
| Administration: |
Drip-on; once daily for 21 days. |
| Result: |
Displayed significantly accelerated healing and increased neovascularization in both aged and diabetic mice model. |
| Animal Model: |
Male Sprague-Dawley rats (180-200 g). |
| Dosage: |
200 mg/kg |
| Administration: |
Intraperitoneal injection; daily for 2 weeks. |
| Result: |
Significantly increased hepatic HIF-1α protein levels, InsR protein levels, as well as Akt/PKB and activated Akt/PKB were significantly higher in the liver. |
|
|---|
| 体内研究 |
Deferoxamine (560.68 mg/per; drip-on; once daily for 21 days) enhances wound healing and increases neovascularization in aged or diabetic mice.
Deferoxamine (200 mg/kg; i.p.; daily for 2 weeks) results in HIF-1α stabilization and increases glucose uptake, hepatic InsR expression, and signaling in vivo.
西域 has not independently confirmed the accuracy of these methods. They are for reference only.
| Animal Model: |
Aged (21-month-old) and diabetic (12-week-old) C57BL/6J mice (excisional wound model). |
| Dosage: |
560.68 mg/per (10 uL of 1 mM) |
| Administration: |
Drip-on; once daily for 21 days. |
| Result: |
Displayed significantly accelerated healing and increased neovascularization in both aged and diabetic mice model. |
| Animal Model: |
Male Sprague-Dawley rats (180-200 g). |
| Dosage: |
200 mg/kg |
| Administration: |
Intraperitoneal injection; daily for 2 weeks. |
| Result: |
Significantly increased hepatic HIF-1α protein levels, InsR protein levels, as well as Akt/PKB and activated Akt/PKB were significantly higher in the liver. |
|
|---|
| 性状 | Solid |
|---|
| 溶解性数据 |
In Vitro:
DMSO : 12.5 mg/mL (22.29 mM; ultrasonic and warming and heat to 60°C)
配制储备液
|
浓度
溶剂体积
质量
|
1 mg |
5 mg |
10 mg |
| 1 mM |
1.7835 mL |
8.9177 mL |
17.8355 mL |
| 5 mM |
0.3567 mL |
1.7835 mL |
3.5671 mL |
| 10 mM |
0.1784 mL |
0.8918 mL |
1.7835 mL |
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。
储备液的保存方式和期限:-80°C, 6 months; -20°C, 1 month。-80°C 储存时,请在 6 个月内使用,-20°C 储存时,请在 1 个月内使用。
In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂:
——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用;
以下溶剂前显示的百
分比是指该溶剂在您配制终溶液中的体积占比;如在配制过程中出现沉淀、析出现象,可以通过加热和/或超声的方式助溶
-
1.
请依序添加每种溶剂: 10% DMSO 40% PEG300 5% Tween-80 45% saline Solubility: ≥ 1.25 mg/mL (2.23 mM); Clear solution
此方案可获得 ≥ 1.25 mg/mL (2.23 mM,饱和度未知) 的澄清溶液。 以 1 mL 工作液为例,取 100 μL 12.5 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀;向上述体系中加入50 μL Tween-80,混合均匀;然后继续加入 450 μL生理盐水定容至 1 mL。
-
2.
请依序添加每种溶剂: 10% DMSO 90% (20% SBE-β-CD in saline) Solubility: ≥ 1.25 mg/mL (2.23 mM); Clear solution
此方案可获得 ≥ 1.25 mg/mL (2.23 mM,饱和度未知) 的澄清溶液。 以 1 mL 工作液为例,取 100 μL 12.5 mg/mL 的澄清 DMSO 储备液加到 900 μL 20% 的 SBE-β-CD 生理盐水水溶液中,混合均匀。
-
3.
请依序添加每种溶剂: 10% DMSO 90% corn oil Solubility: ≥ 1.25 mg/mL (2.23 mM); Clear solution
此方案可获得 ≥ 1.25 mg/mL (2.23 mM,饱和度未知) 的澄清溶液,此方案不适用于实验周期在半个月以上的实验。 以 1 mL 工作液为例,取 100 μL 12.5 mg/mL 的澄清 DMSO 储备液加到 900 μL玉米油中,混合均匀。
*以上所有助溶剂都可在 西域 网站选购。
|
|---|
| 运输条件 |
Room temperature in continental US; may vary elsewhere.
|
|---|
| 储存方式 |
| Powder |
-20°C |
3 years |
|
4°C |
2 years |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
|
|---|
| 参考文献 | |
|---|
 有竞争力的价格
有竞争力的价格 极速物流
极速物流 技术支持
技术支持 现货库存
现货库存

 浙公网安备 33010802013016号
浙公网安备 33010802013016号